Coordination of cellular differentiation, polarity, mitosis and meiosis - New findings from early vertebrate oogenesis
- PMID: 28666956
- PMCID: PMC5623617
- DOI: 10.1016/j.ydbio.2017.06.029
Coordination of cellular differentiation, polarity, mitosis and meiosis - New findings from early vertebrate oogenesis
Abstract
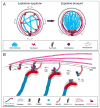
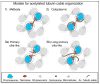
A mechanistic dissection of early oocyte differentiation in vertebrates is key to advancing our knowledge of germline development, reproductive biology, the regulation of meiosis, and all of their associated disorders. Recent advances in the field include breakthroughs in the identification of germline stem cells in Medaka, in the cellular architecture of the germline cyst in mice, in a mechanistic dissection of chromosomal pairing and bouquet formation in meiosis in mice, in tracing oocyte symmetry breaking to the chromosomal bouquet of meiosis in zebrafish, and in the biology of the Balbiani body, a universal oocyte granule. Many of the major events in early oogenesis are universally conserved, and some are co-opted for species-specific needs. The chromosomal events of meiosis are of tremendous consequence to gamete formation and have been extensively studied. New light is now being shed on other aspects of early oocyte differentiation, which were traditionally considered outside the scope of meiosis, and their coordination with meiotic events. The emerging theme is of meiosis as a common groundwork for coordinating multifaceted processes of oocyte differentiation. In an accompanying manuscript we describe methods that allowed for investigations in the zebrafish ovary to contribute to these breakthroughs. Here, we review these advances mostly from the zebrafish and mouse. We discuss oogenesis concepts across established model organisms, and construct an inclusive paradigm for early oocyte differentiation in vertebrates.
Keywords: Animal-vegetal axis; Balbiani body; Centrosome; Chromosomal bouquet; Meiosis; Oocyte polarity; Oogenesis; Symmetry-breaking; Zebrafish.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


References
-
- Albamonte MI, Albamonte MS, Stella I, Zuccardi L, Vitullo AD. The infant and pubertal human ovary: Balbiani’s body-associated VASA expression, immunohistochemical detection of apoptosis-related BCL2 and BAX proteins, and DNA fragmentation. Hum Reprod. 2013;28(3):698–706. doi: 10.1093/humrep/des453. - DOI - PubMed
-
- Barton BR, Hertig AT. Ultrastructure of annulate lamellae in primary oocytes of chimpanzees (Pan troglodytes) Biol Reprod. 1972;6(1):98–108. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

