Functional perturbation of forebrain principal neurons reveals differential effects in novel and well-learned tasks
- PMID: 28666957
- PMCID: PMC5940842
- DOI: 10.1016/j.brainres.2017.06.024
Functional perturbation of forebrain principal neurons reveals differential effects in novel and well-learned tasks
Abstract
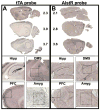
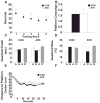
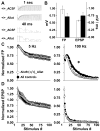
Neural circuits in mammalian brains consist of large numbers of different cell types having different functional properties. To better understand the separate roles of individual neuron types in specific aspects of spatial learning and memory, we perturbed the function of principal neurons in vivo during maze performance or in hippocampal slices during recording of evoked excitatory synaptic potentials. Transgenic mice expressing the Drosophila allatostatin receptor (AlstR) in cortical and hippocampal pyramidal cells were tested on an elevated plus maze, in a Y-maze, and in the Morris water maze. Relative to a control cohort, AlstR-positive mice treated with allatostatin exhibited no difference in open arm dwell time on the elevated plus maze or total number of arm entries in a Y-maze, but displayed reduced spontaneous alternation. When animals received massed or spaced training trials in the Morris water maze, and the peptide was delivered prior to an immediate probe, no effects on performance were observed. When the peptide was delivered during a probe trial performed 24h after seven days of spaced training, allatostatin delivery to AlstR positive mice enhanced direct navigation to the escape platform. Combined, these results suggest that cortical and hippocampal pyramidal neurons are required during spatial decision-making in a novel environment and compete with other neural systems after extended training in a long-term reference memory task. In hippocampal slices collected from AlstR positive animals, allatostatin delivery produced frequency dependent alterations in the Schaffer collateral fiber volley (attenuated accommodation at 100Hz) and excitatory postsynaptic potential (attenuated facilitation at 5Hz). Combined, the neural and behavioral discoveries support the involvement of short-term plasticity of Schaffer collateral axons and synapses during exploration of a novel environment and during initial orientation to a goal in a well-learned setting.
Keywords: Allatostatin receptor; GIRK; Hippocampus; Pyramidal neuron; Spatial learning and memory.
Copyright © 2017 Elsevier B.V. All rights reserved.
Conflict of interest statement
There are no conflicts of interest for any authors of this study.
Figures



Similar articles
-
Transgenic silencing of neurons in the mammalian brain by expression of the allatostatin receptor (AlstR).J Neurophysiol. 2009 Oct;102(4):2554-62. doi: 10.1152/jn.00480.2009. Epub 2009 Aug 19. J Neurophysiol. 2009. PMID: 19692509 Free PMC article.
-
Relaxin-3 inputs target hippocampal interneurons and deletion of hilar relaxin-3 receptors in "floxed-RXFP3" mice impairs spatial memory.Hippocampus. 2017 May;27(5):529-546. doi: 10.1002/hipo.22709. Epub 2017 Jan 31. Hippocampus. 2017. PMID: 28100033
-
Intracellular correlates of spatial memory acquisition in hippocampal slices: long-term disinhibition of CA1 pyramidal cells.J Neurophysiol. 2001 Aug;86(2):881-99. doi: 10.1152/jn.2001.86.2.881. J Neurophysiol. 2001. PMID: 11495958
-
[Possible mechanisms for orexin effects on the functioning of the hippocampus and spatial learning (analytical review)].Zh Vyssh Nerv Deiat Im I P Pavlova. 2012 Jul-Aug;62(4):389-400. Zh Vyssh Nerv Deiat Im I P Pavlova. 2012. PMID: 23035556 Review. Russian.
-
Age-related biophysical alterations of hippocampal pyramidal neurons: implications for learning and memory.Ageing Res Rev. 2002 Apr;1(2):181-207. doi: 10.1016/s1568-1637(01)00009-5. Ageing Res Rev. 2002. PMID: 12039438 Review.
Cited by
-
Propionic acid ameliorates cognitive function through immunomodulatory effects on Th17 cells in perioperative neurocognitive disorders.Heliyon. 2024 Mar 27;10(8):e28817. doi: 10.1016/j.heliyon.2024.e28817. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38699705 Free PMC article.
References
-
- Albani SH, Andrawis MM, Abella RJH, Fulghum JT, Vafamand N, Dumas TC. Behavior in the elevated plus maze is differentially affected by testing conditions in rats under and over three weeks of age. Front Behav Neurosci. 2015;9:31. http://dx.doi.org/10.3389/fnbeh.2015.00031. - DOI - PMC - PubMed
-
- Barber DM, Schönberger M, Burgstaller J, Levitz J, Weaver CD, Isacoff EY, Baier H, Trauner D. Optical control of neuronal activity using a light-operated GIRK channel opener (LOGO) Chem Sci. 2016;7(3):2347–2352. http://dx.doi.org/10.1039/C5SC04084A. - DOI - PMC - PubMed
-
- Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn Sci. 2011;15:592–600. http://dx.doi.org/10.1016/j.tics.2011.10.003. - DOI - PMC - PubMed
-
- Blair MG, Nguyen NNQ, Albani SH, L’Etoile MM, Andrawis MM, Owen LM, Oliveira RF, Johnson MW, Purvis DL, Sanders EM, Stoneham ET, Xu H, Dumas TC. Developmental changes in structural and functional properties of hippocampal AMPARs parallels the emergence of deliberative spatial navigation in juvenile rats. J Neurosci. 2013;33:12218–12228. http://dx.doi.org/10.1523/JNEUROSCI.4827-12.2013. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

