Cross-linking of dicyclotyrosine by the cytochrome P450 enzyme CYP121 from Mycobacterium tuberculosis proceeds through a catalytic shunt pathway
- PMID: 28667013
- PMCID: PMC5569305
- DOI: 10.1074/jbc.M117.794099
Cross-linking of dicyclotyrosine by the cytochrome P450 enzyme CYP121 from Mycobacterium tuberculosis proceeds through a catalytic shunt pathway
Abstract
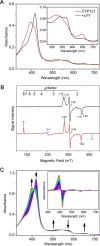
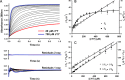
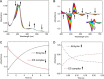
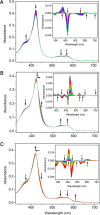
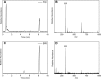
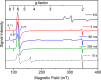
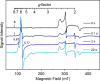
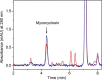
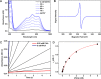
CYP121, the cytochrome P450 enzyme in Mycobacterium tuberculosis that catalyzes a single intramolecular C-C cross-linking reaction in the biosynthesis of mycocyclosin, is crucial for the viability of this pathogen. This C-C coupling reaction represents an expansion of the activities carried out by P450 enzymes distinct from oxygen insertion. Although the traditional mechanism for P450 enzymes has been well studied, it is unclear whether CYP121 follows the general P450 mechanism or uses a different catalytic strategy for generating an iron-bound oxidant. To gain mechanistic insight into the CYP121-catalyzed reaction, we tested the peroxide shunt pathway by using rapid kinetic techniques to monitor the enzyme activity with its substrate dicyclotyrosine (cYY) and observed the formation of the cross-linked product mycocyclosin by LC-MS. In stopped-flow experiments, we observed that cYY binding to CYP121 proceeds in a two-step process, and EPR spectroscopy indicates that the binding induces active site reorganization and uniformity. Using rapid freeze-quenching EPR, we observed the formation of a high-spin intermediate upon the addition of peracetic acid to the enzyme-substrate complex. This intermediate exhibits a high-spin (S = 5/2) signal with g values of 2.00, 5.77, and 6.87. Likewise, iodosylbenzene could also produce mycocyclosin, implicating compound I as the initial oxidizing species. Moreover, we also demonstrated that CYP121 performs a standard peroxidase type of reaction by observing substrate-based radicals. On the basis of these results, we propose plausible free radical-based mechanisms for the C-C bond coupling reaction.
Keywords: C–C bond coupling; cyclodipeptide; cytochrome P450; diradical; electron transfer; enzyme kinetics; metabolism; oxygen activation; tuberculosis.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Identification and structural basis of the reaction catalyzed by CYP121, an essential cytochrome P450 in Mycobacterium tuberculosis.Proc Natl Acad Sci U S A. 2009 May 5;106(18):7426-31. doi: 10.1073/pnas.0812191106. Epub 2009 Apr 22. Proc Natl Acad Sci U S A. 2009. PMID: 19416919 Free PMC article.
-
Substrate and reaction specificity of Mycobacterium tuberculosis cytochrome P450 CYP121: insights from biochemical studies and crystal structures.J Biol Chem. 2013 Jun 14;288(24):17347-59. doi: 10.1074/jbc.M112.443853. Epub 2013 Apr 25. J Biol Chem. 2013. PMID: 23620594 Free PMC article.
-
Acetylene containing cyclo(L-Tyr-L-Tyr)-analogs as mechanism-based inhibitors of CYP121A1 from Mycobacterium tuberculosis.Biochem Pharmacol. 2020 Jul;177:113938. doi: 10.1016/j.bcp.2020.113938. Epub 2020 Mar 26. Biochem Pharmacol. 2020. PMID: 32224137
-
CYP121, CYP51 and associated redox systems in Mycobacterium tuberculosis: towards deconvoluting enzymology of P450 systems in a human pathogen.Biochem Soc Trans. 2006 Dec;34(Pt 6):1178-82. doi: 10.1042/BST0341178. Biochem Soc Trans. 2006. PMID: 17073780 Review.
-
Differential behavior of the sub-sites of cytochrome 450 active site in binding of substrates, and products (implications for coupling/uncoupling).Biochim Biophys Acta. 2007 Mar;1770(3):360-75. doi: 10.1016/j.bbagen.2006.09.018. Epub 2006 Oct 5. Biochim Biophys Acta. 2007. PMID: 17134838 Review.
Cited by
-
Probing Ligand Exchange in the P450 Enzyme CYP121 from Mycobacterium tuberculosis: Dynamic Equilibrium of the Distal Heme Ligand as a Function of pH and Temperature.J Am Chem Soc. 2017 Dec 6;139(48):17484-17499. doi: 10.1021/jacs.7b08911. Epub 2017 Nov 20. J Am Chem Soc. 2017. PMID: 29090577 Free PMC article.
-
Mapping and Exploiting the Promiscuity of OxyB toward the Biocatalytic Production of Vancomycin Aglycone Variants.ACS Catal. 2020 Aug 21;10(16):9287-9298. doi: 10.1021/acscatal.0c01719. Epub 2020 Jul 9. ACS Catal. 2020. PMID: 34422446 Free PMC article.
-
Mechanistic analysis of carbon-carbon bond formation by deoxypodophyllotoxin synthase.Proc Natl Acad Sci U S A. 2022 Jan 4;119(1):e2113770119. doi: 10.1073/pnas.2113770119. Proc Natl Acad Sci U S A. 2022. PMID: 34969844 Free PMC article.
-
Characterization of the nonheme iron center of cysteamine dioxygenase and its interaction with substrates.J Biol Chem. 2020 Aug 14;295(33):11789-11802. doi: 10.1074/jbc.RA120.013915. Epub 2020 Jun 28. J Biol Chem. 2020. PMID: 32601061 Free PMC article.
-
Depicting the proton relay network in human aromatase: New insights into the role of the alcohol-acid pair.Protein Sci. 2022 Sep;31(9):e4389. doi: 10.1002/pro.4389. Protein Sci. 2022. PMID: 36040260 Free PMC article.
References
-
- World Health Organization (2016) Global Tuberculosis Report 2016, World Health Organization, Geneva, Switzerland
-
- Matsumoto M., Hashizume H., Tsubouchi H., Sasaki H., Itotani M., Kuroda H., Tomishige T., Kawasaki M., and Komatsu M. (2007) Screening for novel antituberculosis agents that are effective against multidrug resistant tuberculosis. Curr. Top. Med. Chem. 7, 499–507 - PubMed
-
- Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E. 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., et al. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 - PubMed
-
- Souter A., McLean K. J., Smith W. E., and Munro A. W. (2000) The genome sequence of Mycobacterium tuberculosis reveals cytochromes P450 as novel anti-TB drug targets. J. Chem. Technol. Biotechnol. 75, 933–941
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

