Decreased Expression of Connexin 43 Blunts the Progression of Experimental GN
- PMID: 28667079
- PMCID: PMC5619962
- DOI: 10.1681/ASN.2016111211
Decreased Expression of Connexin 43 Blunts the Progression of Experimental GN
Abstract
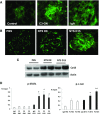
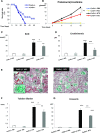
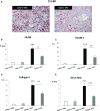
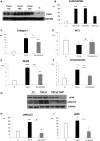
GN refers to a variety of renal pathologies that often progress to ESRD, but the molecular mechanisms underlying this progression remain incompletely characterized. Here, we determined whether dysregulated expression of the gap junction protein connexin 43, which has been observed in the progression of renal disease, contributes to GN progression. Immunostaining revealed de novo expression of connexin 43 in damaged glomeruli in patients with glomerular diseases as well as in mice after induction of experimental GN. Notably, 2 weeks after the induction of GN with nephrotoxic serum, mice with a heterozygous deletion of the connexin 43 gene (connexin 43+/-) had proteinuria, BUN, and serum creatinine levels significantly lower than those of wild-type animals. Additionally, the connexin 43+/- mice showed less crescent formation, tubular dilation, monocyte infiltration, and interstitial renal fibrosis. Treatment of cultured podocytes with connexin 43-specific blocking peptides attenuated TGF-β-induced cytoskeletal and morphologic changes and apoptosis as did treatment with the purinergic blocker suramin. Finally, therapeutic treatment of GN mice with connexin 43-specific antisense oligodeoxynucleotide improved functional and structural renal parameters. These findings suggest that crosstalk between connexin 43 and purinergic signaling contributes to podocyte damage in GN. Given that this protein is highly induced in individuals with glomerular diseases, connexin 43 may be a novel target for therapeutic treatment of GN.
Keywords: connexins; glomerular disease; podocyte; renal fibrosis.
Copyright © 2017 by the American Society of Nephrology.
Figures





Comment in
-
Cell biology: Connexin connections in GN.Nat Rev Nephrol. 2017 Sep;13(9):514. doi: 10.1038/nrneph.2017.106. Epub 2017 Jul 17. Nat Rev Nephrol. 2017. PMID: 28713167 No abstract available.
Similar articles
-
Signaling through cAMP-Epac1 induces metabolic reprogramming to protect podocytes in glomerulonephritis.Kidney Int. 2024 Sep;106(3):450-469. doi: 10.1016/j.kint.2024.05.010. Epub 2024 May 29. Kidney Int. 2024. PMID: 38821447
-
DNA fragmentation in chronic glomerulonephritis: an immunohistological analysis.Nephron Clin Pract. 2007;105(1):c18-28. doi: 10.1159/000096981. Epub 2006 Nov 15. Nephron Clin Pract. 2007. PMID: 17114899
-
Activation of Notch3 in Glomeruli Promotes the Development of Rapidly Progressive Renal Disease.J Am Soc Nephrol. 2015 Jul;26(7):1561-75. doi: 10.1681/ASN.2013090968. Epub 2014 Nov 24. J Am Soc Nephrol. 2015. PMID: 25421557 Free PMC article.
-
[The role of tubulointerstitial changes in progression of kidney function failure in patients with chronic glomerulonephritis (GN)].Przegl Lek. 1996;53(5):443-53. Przegl Lek. 1996. PMID: 8754411 Review. Polish.
-
Connexin 43: a New Therapeutic Target Against Chronic Kidney Disease.Cell Physiol Biochem. 2018;49(3):985. doi: 10.1159/000493230. Epub 2018 Sep 7. Cell Physiol Biochem. 2018. PMID: 30196298 Review.
Cited by
-
Connexin 43: A Target for the Treatment of Inflammation in Secondary Complications of the Kidney and Eye in Diabetes.Int J Mol Sci. 2022 Jan 6;23(2):600. doi: 10.3390/ijms23020600. Int J Mol Sci. 2022. PMID: 35054783 Free PMC article. Review.
-
Genetic deletion of connexin 37 causes polyuria and polydipsia.PLoS One. 2020 Dec 17;15(12):e0244251. doi: 10.1371/journal.pone.0244251. eCollection 2020. PLoS One. 2020. PMID: 33332450 Free PMC article.
-
Effects of Suramin on Polycystic Kidney Disease in a Mouse Model of Polycystin-1 Deficiency.Int J Mol Sci. 2022 Jul 31;23(15):8499. doi: 10.3390/ijms23158499. Int J Mol Sci. 2022. PMID: 35955634 Free PMC article.
-
The Role of Connexin 43 in Renal Disease: Insights from In Vivo Models of Experimental Nephropathy.Int J Mol Sci. 2022 Oct 28;23(21):13090. doi: 10.3390/ijms232113090. Int J Mol Sci. 2022. PMID: 36361888 Free PMC article. Review.
-
The sGC Activator Runcaciguat Has Kidney Protective Effects and Prevents a Decline of Kidney Function in ZSF1 Rats.Int J Mol Sci. 2023 Aug 25;24(17):13226. doi: 10.3390/ijms241713226. Int J Mol Sci. 2023. PMID: 37686032 Free PMC article.
References
-
- Chadban SJ, Atkins RC: Glomerulonephritis. Lancet 365: 1797–1806, 2005 - PubMed
-
- Mathieson PW: Glomerulonephritis. Semin Immunopathol 29: 315–316, 2007 - PubMed
-
- Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

