CONSORT 2010 statement: extension checklist for reporting within person randomised trials
- PMID: 28667088
- PMCID: PMC5492474
- DOI: 10.1136/bmj.j2835
CONSORT 2010 statement: extension checklist for reporting within person randomised trials
Abstract
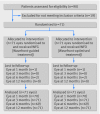
Evidence shows that the quality of reporting of randomised controlled trials (RCTs) is not optimal. The lack of transparent reporting impedes readers from judging the reliability and validity of trial findings and researchers from extracting information for systematic reviews and results in research waste. The Consolidated Standards of Reporting Trials (CONSORT) statement was developed to improve the reporting of RCTs. Within person trials are used for conditions that can affect two or more body sites, and are a useful and efficient tool because the comparisons between interventions are within people. Such trials are most commonly conducted in ophthalmology, dentistry, and dermatology. The reporting of within person trials has, however, been variable and incomplete, hindering their use in clinical decision making and by future researchers. This document presents the CONSORT extension to within person trials. It aims to facilitate the reporting of these trials. It extends 16 items of the CONSORT 2010 checklist and introduces a modified flowchart and baseline table to enhance transparency. Examples of good reporting and evidence based rationale for CONSORT within person checklist items are provided.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
