Molybdenum-Based Diazotrophy in a Sphagnum Peatland in Northern Minnesota
- PMID: 28667112
- PMCID: PMC5561275
- DOI: 10.1128/AEM.01174-17
Molybdenum-Based Diazotrophy in a Sphagnum Peatland in Northern Minnesota
Abstract
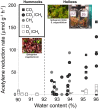
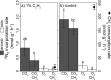
Microbial N2 fixation (diazotrophy) represents an important nitrogen source to oligotrophic peatland ecosystems, which are important sinks for atmospheric CO2 and are susceptible to the changing climate. The objectives of this study were (i) to determine the active microbial group and type of nitrogenase mediating diazotrophy in an ombrotrophic Sphagnum-dominated peat bog (the S1 peat bog, Marcell Experimental Forest, Minnesota, USA); and (ii) to determine the effect of environmental parameters (light, O2, CO2, and CH4) on potential rates of diazotrophy measured by acetylene (C2H2) reduction and 15N2 incorporation. A molecular analysis of metabolically active microbial communities suggested that diazotrophy in surface peat was primarily mediated by Alphaproteobacteria (Bradyrhizobiaceae and Beijerinckiaceae). Despite higher concentrations of dissolved vanadium ([V] 11 nM) than molybdenum ([Mo] 3 nM) in surface peat, a combination of metagenomic, amplicon sequencing, and activity measurements indicated that Mo-containing nitrogenases dominate over the V-containing form. Acetylene reduction was only detected in surface peat exposed to light, with the highest rates observed in peat collected from hollows with the highest water contents. Incorporation of 15N2 was suppressed 90% by O2 and 55% by C2H2 and was unaffected by CH4 and CO2 amendments. These results suggest that peatland diazotrophy is mediated by a combination of C2H2-sensitive and C2H2-insensitive microbes that are more active at low concentrations of O2 and show similar activity at high and low concentrations of CH4 IMPORTANCE Previous studies indicate that diazotrophy provides an important nitrogen source and is linked to methanotrophy in Sphagnum-dominated peatlands. However, the environmental controls and enzymatic pathways of peatland diazotrophy, as well as the metabolically active microbial populations that catalyze this process, remain in question. Our findings indicate that oxygen levels and photosynthetic activity override low nutrient availability in limiting diazotrophy and that members of the Alphaproteobacteria (Rhizobiales) catalyze this process at the bog surface using the molybdenum-based form of the nitrogenase enzyme.
Keywords: Alphaproteobacteria; Sphagnum; acetylene; diazotrophy; methanotrophs; molybdenum; nitrogen cycle enzymes; nitrogen fixation; peatland; vanadium.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
The influence of oxygen and methane on nitrogen fixation in subarctic Sphagnum mosses.AMB Express. 2018 May 5;8(1):76. doi: 10.1186/s13568-018-0607-2. AMB Express. 2018. PMID: 29730829 Free PMC article.
-
Climate drivers alter nitrogen availability in surface peat and decouple N2 fixation from CH4 oxidation in the Sphagnum moss microbiome.Glob Chang Biol. 2023 Jun;29(11):3159-3176. doi: 10.1111/gcb.16651. Epub 2023 Mar 31. Glob Chang Biol. 2023. PMID: 36999440
-
Microbial nitrogen fixation and methane oxidation are strongly enhanced by light in Sphagnum mosses.AMB Express. 2020 Mar 31;10(1):61. doi: 10.1186/s13568-020-00994-9. AMB Express. 2020. PMID: 32236738 Free PMC article.
-
[Methanotrophic bacteria of acid sphagnum bogs].Mikrobiologiia. 2002 Nov-Dec;71(6):741-54. Mikrobiologiia. 2002. PMID: 12526194 Review. Russian.
-
The Sphagnum microbiome: new insights from an ancient plant lineage.New Phytol. 2016 Jul;211(1):57-64. doi: 10.1111/nph.13993. Epub 2016 May 13. New Phytol. 2016. PMID: 27173909 Review.
Cited by
-
The influence of oxygen and methane on nitrogen fixation in subarctic Sphagnum mosses.AMB Express. 2018 May 5;8(1):76. doi: 10.1186/s13568-018-0607-2. AMB Express. 2018. PMID: 29730829 Free PMC article.
-
Minnesota peat viromes reveal terrestrial and aquatic niche partitioning for local and global viral populations.Microbiome. 2021 Nov 26;9(1):233. doi: 10.1186/s40168-021-01156-0. Microbiome. 2021. PMID: 34836550 Free PMC article.
-
Experimental warming alters the community composition, diversity, and N2 fixation activity of peat moss (Sphagnum fallax) microbiomes.Glob Chang Biol. 2019 Sep;25(9):2993-3004. doi: 10.1111/gcb.14715. Epub 2019 Jul 2. Glob Chang Biol. 2019. PMID: 31148286 Free PMC article.
-
Host Species-Microbiome Interactions Contribute to Sphagnum Moss Growth Acclimation to Warming.Glob Chang Biol. 2025 Feb;31(2):e70066. doi: 10.1111/gcb.70066. Glob Chang Biol. 2025. PMID: 39968863 Free PMC article.
-
Diazotroph Community Characterization via a High-Throughput nifH Amplicon Sequencing and Analysis Pipeline.Appl Environ Microbiol. 2018 Jan 31;84(4):e01512-17. doi: 10.1128/AEM.01512-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29180374 Free PMC article.
References
-
- Gorham E, Lehman C, Dyke A, Clymo D, Janssens J. 2012. Long-term carbon sequestration in North American peatlands. Quat Sci Rev 58:1–14. doi:10.1016/j.quascirev.2012.09.018. - DOI
-
- Limpens J, Berendse F, Blodau C, Canadell J, Freeman C, Holden J, Roulet N, Rydin H, Schaepman-Strub G. 2008. Peatlands and the carbon cycle: from local processes to global implications—a synthesis. Biogeosciences 5:1475–1491. doi:10.5194/bg-5-1475-2008. - DOI
-
- Yu Z. 2012. Northern peatland carbon stocks and dynamics: a review. Biogeosciences 9:4071–4085. doi:10.5194/bg-9-4071-2012. - DOI
-
- Clymo R. 1984. The limits to peat bog growth. Philos Trans R Soc Lond B Biol Sci 303:605–654. doi:10.1098/rstb.1984.0002. - DOI
-
- Clymo R, Hayward P. 1982. The ecology of Sphagnum, p 229–289. In Smith AJE. (ed), Bryophyte ecology. Chapman and Hall, London, United Kingdom.
LinkOut - more resources
Full Text Sources
Other Literature Sources

