Distinct VASP tetramers synergize in the processive elongation of individual actin filaments from clustered arrays
- PMID: 28667124
- PMCID: PMC5530675
- DOI: 10.1073/pnas.1703145114
Distinct VASP tetramers synergize in the processive elongation of individual actin filaments from clustered arrays
Abstract
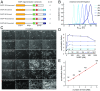
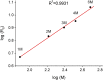
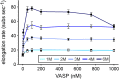
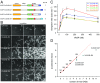
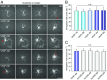
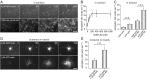
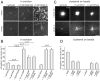
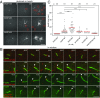
Ena/VASP proteins act as actin polymerases that drive the processive elongation of filament barbed ends in membrane protrusions or at the surface of bacterial pathogens. Based on previous analyses of fast and slow elongating VASP proteins by in vitro total internal reflection fluorescence microscopy (TIRFM) and kinetic and thermodynamic measurements, we established a kinetic model of Ena/VASP-mediated actin filament elongation. At steady state, it entails that tetrameric VASP uses one of its arms to processively track growing filament barbed ends while three G-actin-binding sites (GABs) on other arms are available to recruit and deliver monomers to the filament tip, suggesting that VASP operates as a single tetramer in solution or when clustered on a surface, albeit processivity and resistance toward capping protein (CP) differ dramatically between both conditions. Here, we tested the model by variation of the oligomerization state and by increase of the number of GABs on individual polypeptide chains. In excellent agreement with model predictions, we show that in solution the rates of filament elongation directly correlate with the number of free GABs. Strikingly, however, irrespective of the oligomerization state or presence of additional GABs, filament elongation on a surface invariably proceeded with the same rate as with the VASP tetramer, demonstrating that adjacent VASP molecules synergize in the elongation of a single filament. Additionally, we reveal that actin ATP hydrolysis is not required for VASP-mediated filament assembly. Finally, we show evidence for the requirement of VASP to form tetramers and provide an amended model of processive VASP-mediated actin assembly in clustered arrays.
Keywords: Ena/VASP; TIRF; actin; cluster; formin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bugyi B, Carlier M-F. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470. - PubMed
-
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–263. - PubMed
-
- Krause M, Gautreau A. Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–590. - PubMed
-
- Le Clainche C, Carlier M-F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol Rev. 2008;88:489–513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

