Fam64a is a novel cell cycle promoter of hypoxic fetal cardiomyocytes in mice
- PMID: 28667270
- PMCID: PMC5493652
- DOI: 10.1038/s41598-017-04823-1
Fam64a is a novel cell cycle promoter of hypoxic fetal cardiomyocytes in mice
Abstract
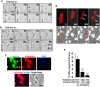
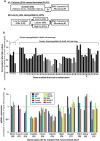
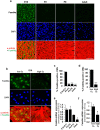
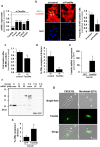
Fetal cardiomyocytes actively proliferate to form the primitive heart in utero in mammals, but they stop dividing shortly after birth. The identification of essential molecules maintaining this active cardiomyocyte proliferation is indispensable for potential adult heart regeneration. A recent study has shown that this proliferation depends on a low fetal oxygen condition before the onset of breathing at birth. We have established an isolation protocol for mouse fetal cardiomyocytes, performed under strict low oxygen conditions to mimic the intrauterine environment, that gives the highest proliferative activities thus far reported. Oxygen exposure during isolation/culture markedly inhibited cell division and repressed cell cycle-promoting genes, and subsequent genome-wide analysis identified Fam64a as a novel regulatory molecule. Fam64a was abundantly expressed in hypoxic fetal cardiomyocyte nuclei, but this expression was drastically repressed by oxygen exposure, and in postnatal cardiomyocytes following the onset of breathing and the resulting elevation of oxygen tension. Fam64a knockdown inhibited and its overexpression enhanced cardiomyocyte proliferation. Expression of a non-degradable Fam64a mutant suggested that optimum Fam64a expression and subsequent degradation by anaphase-promoting complex/cyclosome (APC/C) during the metaphase-to-anaphase transition are required for fetal cardiomyocyte division. We propose that Fam64a is a novel cell cycle promoter of hypoxic fetal cardiomyocytes in mice.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

