Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus
- PMID: 28667908
- PMCID: PMC5493838
- DOI: 10.1016/j.redox.2017.06.010
Transcription factor NRF2 controls the fate of neural stem cells in the subgranular zone of the hippocampus
Abstract
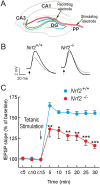
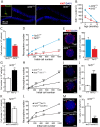
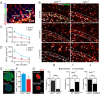
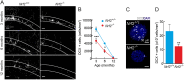
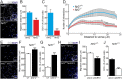
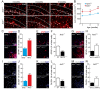
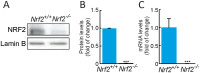
Neural stem/progenitor cells (NSPCs) located at the subgranular zone (SGZ) of the hippocampus participate in the maintenance of synaptic networks that ensure cognitive functions during life. Although it is known that this neurogenic niche losses activity with oxidative stress and ageing, the molecular events involved in its regulation are largely unknown. Here, we studied the role of transcription factor Nuclear Factor-Erythroid 2-Related Factor 2 (NRF2) in the control of NSPCs destinies in the SGZ. We first describe that NRF2-knockout (Nrf2-/-) mice exhibit impaired long term potentiation, a function that requires integrity of the SGZ, therefore suggesting a cognitive deficit that might be linked to hippocampal neurogenesis. Then, we found a reduction in NSCs from birth to adulthood that was exacerbated in Nrf2-/- vs. Nrf2+/+ mice. The clonogenic and proliferative capacity of SGZ-derived NSPCs from newborn and 3-month-old Nrf2-/- mice was severely reduced as determined in neurosphere cultures. Nrf2-deficiency also impaired neuronal differentiation both the SGZ, and in neurosphere differentiation assays, leading to an abnormal production of astrocytes and oligodendrocytes vs. neurons. Rescue of Nrf2-/- NSPCs by ectopic expression of NRF2 attenuated the alterations in clonogenic, proliferative and differentiating capacity of hippocampal NSPCs. In turn, knockdown of the NRF2 gene in wild type NSPCs reproduced the data obtained with Nrf2-/- NSPCs. Our findings demonstrate the importance of NRF2 in the maintenance of proper proliferation and differentiation rates of hippocampal NSPCs and suggest that interventions to up-regulate NRF2 might provide a mechanism to preserve the neurogenic functionality of the hippocampus.
Keywords: Aging; Hippocampal neurogenesis; NRF2; Neural stem cells; Oxidative stress; Subgranular zone.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
A Role for Nrf2 Expression in Defining the Aging of Hippocampal Neural Stem Cells.Cell Transplant. 2018 Apr;27(4):589-606. doi: 10.1177/0963689718774030. Epub 2018 Jun 5. Cell Transplant. 2018. PMID: 29871525 Free PMC article.
-
Neurogenic differentiation by hippocampal neural stem and progenitor cells is biased by NFIX expression.Development. 2018 Feb 7;145(3):dev155689. doi: 10.1242/dev.155689. Development. 2018. PMID: 29437824
-
CD44 Transmembrane Receptor and Hyaluronan Regulate Adult Hippocampal Neural Stem Cell Quiescence and Differentiation.J Biol Chem. 2017 Mar 17;292(11):4434-4445. doi: 10.1074/jbc.M116.774109. Epub 2017 Jan 31. J Biol Chem. 2017. PMID: 28154169 Free PMC article.
-
Age-dependent decline in neurogenesis of the hippocampus and extracellular nucleotides.Hum Cell. 2019 Apr;32(2):88-94. doi: 10.1007/s13577-019-00241-9. Epub 2019 Feb 7. Hum Cell. 2019. PMID: 30730038 Review.
-
The role of the N-methyl-D-aspartate receptor in the proliferation of adult hippocampal neural stem and precursor cells.Sci China Life Sci. 2014 Apr;57(4):403-11. doi: 10.1007/s11427-014-4637-y. Epub 2014 Mar 14. Sci China Life Sci. 2014. PMID: 24777380 Review.
Cited by
-
Ependymal and Neural Stem Cells of Adult Molly Fish (Poecilia sphenops, Valenciennes, 1846) Brain: Histomorphometry, Immunohistochemical, and Ultrastructural Studies.Cells. 2022 Aug 27;11(17):2659. doi: 10.3390/cells11172659. Cells. 2022. PMID: 36078068 Free PMC article.
-
NRF2 and the Moirai: Life and Death Decisions on Cell Fates.Antioxid Redox Signal. 2023 Mar;38(7-9):684-708. doi: 10.1089/ars.2022.0200. Epub 2023 Feb 22. Antioxid Redox Signal. 2023. PMID: 36509429 Free PMC article. Review.
-
Nrf2 deletion results in impaired performance in memory tasks and hyperactivity in mature and aged mice.Brain Res. 2018 Dec 15;1701:103-111. doi: 10.1016/j.brainres.2018.08.033. Epub 2018 Sep 5. Brain Res. 2018. PMID: 30194014 Free PMC article.
-
Melatonin Ameliorates Valproic Acid-Induced Neurogenesis Impairment: The Role of Oxidative Stress in Adult Rats.Oxid Med Cell Longev. 2021 Nov 12;2021:9997582. doi: 10.1155/2021/9997582. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34804374 Free PMC article.
-
Role of Nrf2 in Synaptic Plasticity and Memory in Alzheimer's Disease.Cells. 2021 Jul 25;10(8):1884. doi: 10.3390/cells10081884. Cells. 2021. PMID: 34440653 Free PMC article. Review.
References
-
- Rojo A.I., Innamorato N.G., Martin-Moreno A.M., De Ceballos M.L., Yamamoto M., Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58(5):588–598. - PubMed
-
- Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39(4):199–218. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

