Utility of somatosensory evoked potentials in the assessment of response to IVIG in a long-lasting case of chronic immune sensory polyradiculopathy
- PMID: 28668085
- PMCID: PMC5494125
- DOI: 10.1186/s12883-017-0906-2
Utility of somatosensory evoked potentials in the assessment of response to IVIG in a long-lasting case of chronic immune sensory polyradiculopathy
Abstract
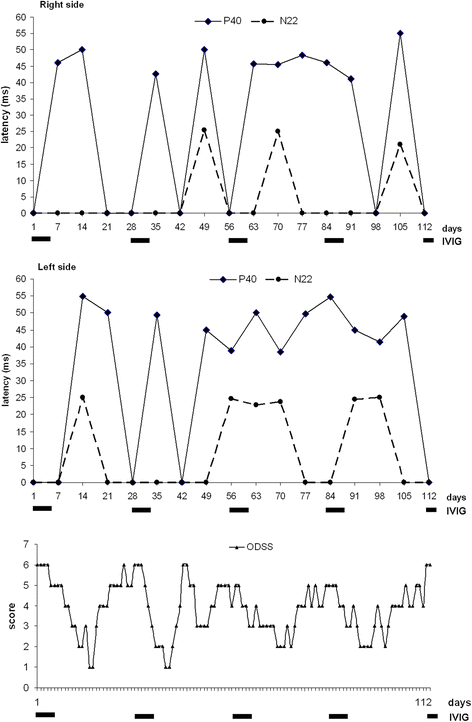
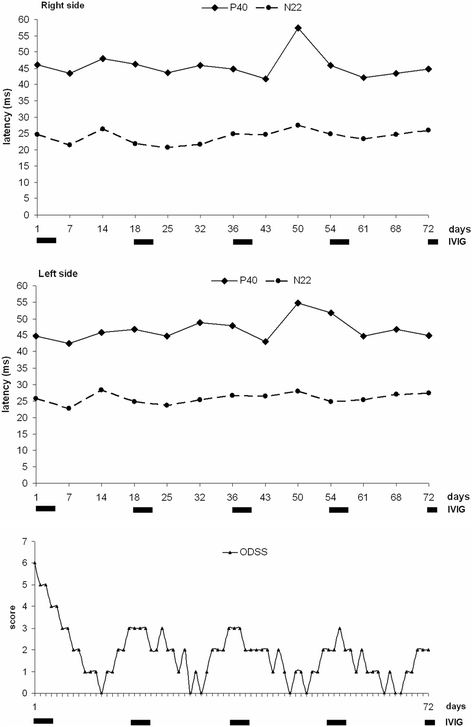
Background: Chronic immune sensory polyradiculopathy (CISP) identifies a progressive acquired peripheral dysimmune neuropathy recognized as a chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) variant. We describe a young woman with a thirteen-year history of CISP with a belated variable response to intravenous immunoglobulin (IVIG) and an almost erratic anticipation of symptoms between IVIG cycles. The association of IVIG and corticosteroids, immunosuppressants, plasmapheresis, did not lead to clinical improvement and was characterized by significant side effects. We evaluated a combined clinical and somatosensory evoked potentials (SSEPs) approach aimed to identify possible predictive parameters concerning the effect and duration of each IVIG administration. Neurologic disability was evaluated using INCAT - Overall Disability Sum Score (INCAT-ODSS).
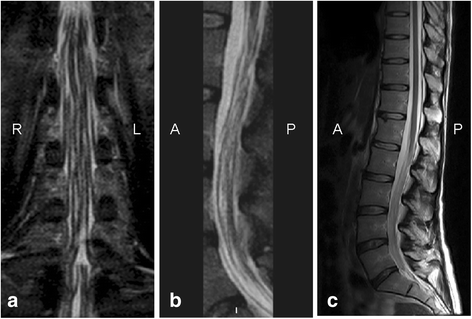
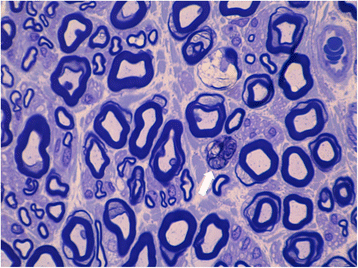
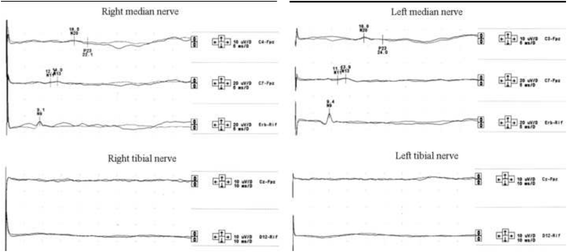
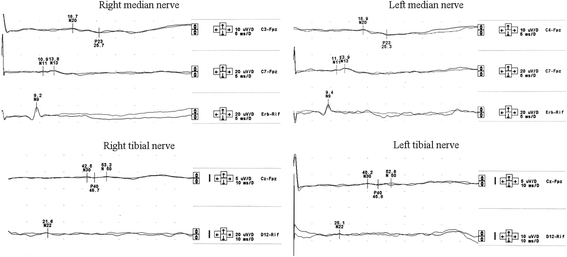
Case presentation: A 30-year-old woman presented on 2004 for the subacute onset of asymmetric paresthesias in the lower limbs over the previous six months. The symptoms had been relapsing-remitting during the first four months, followed by a slow progression, resulting in limbs ataxia and a progressive gait disturbance requiring Canadian crutches. Motor and sensory nerve conduction studies and electromyographic evaluation were into normal limits. Median SSEPs were normal, while tibial SSEPs were characterised by the bilateral absence of both lumbar and cortical responses. Cerebrospinal fluid detected an increased protein concentration, while spinal MRI showed a pronounced thickening of the sacral nerve roots, together with a tube-shaped enlargement. These findings led to the diagnosis of CISP and the patient was treated with IVIG reaching a stable remission over the following 9 years. In early 2014, the patient began to show a variable response to treatment with erratic anticipation of sensory disturbances, and a more pronounced walking disability: corticosteroids, plasmapheresis, mycophenolate mofetil and cyclophosphamide were uneffective and burdened by relevant side effects. To better assess the response to IVIG in terms of time-effect, consistency and duration, we have combined a scheduled clinical and SSEPs evaluation during and after each IVIG cycle.
Conclusions: The correlation between the neurophysiological data and the INCAT-ODSS scores has allowed the modulation of IVIG cycles with a significant reduction of the clinical fluctuations and disability. SSEPs may therefore represent an useful and recommended additional aid for the treatment schedule of this rare clinical form.
Keywords: Chronic immune sensory polyradiculopathy; Chronic inflammatory demyelinating polyneuropathy; INCAT - Overall disability sum score; Intravenous immunoglobulin; Somatosensory evoked potentials.
Conflict of interest statement
Ethics approval and consent to participate
“not applicable”.
Consent for publication
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The Authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Reynolds J, Sachs G, Stavros K. Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): clinical features, diagnosis, and current treatment strategies. R I Med J (2013) 2016;99(12):32–35. - PubMed
-
- Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies; Peripheral nerve society. European Federation of Neurological Societies/peripheral nerve society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the peripheral nerve society - first revision. Eur J Neurol. 2010;17(3):356–363. doi: 10.1111/j.1468-1331.2009.02930.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

