Dissecting the role of non-coding RNAs in the accumulation of amyloid and tau neuropathologies in Alzheimer's disease
- PMID: 28668092
- PMCID: PMC5494142
- DOI: 10.1186/s13024-017-0191-y
Dissecting the role of non-coding RNAs in the accumulation of amyloid and tau neuropathologies in Alzheimer's disease
Abstract
Background: Given multiple studies of brain microRNA (miRNA) in relation to Alzheimer's disease (AD) with few consistent results and the heterogeneity of this disease, the objective of this study was to explore their mechanism by evaluating their relation to different elements of Alzheimer's disease pathology, confounding factors and mRNA expression data from the same subjects in the same brain region.
Methods: We report analyses of expression profiling of miRNA (n = 700 subjects) and lincRNA (n = 540 subjects) from the dorsolateral prefrontal cortex of individuals participating in two longitudinal cohort studies of aging.
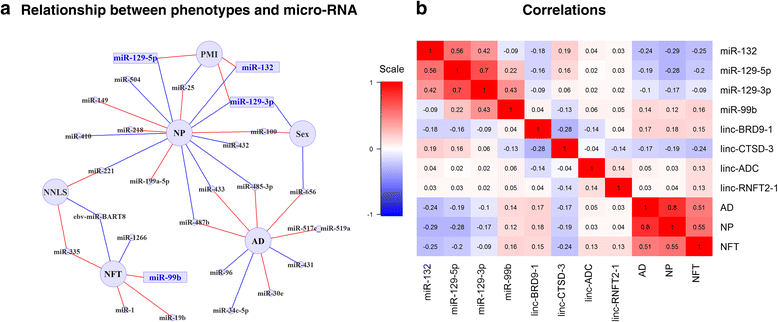
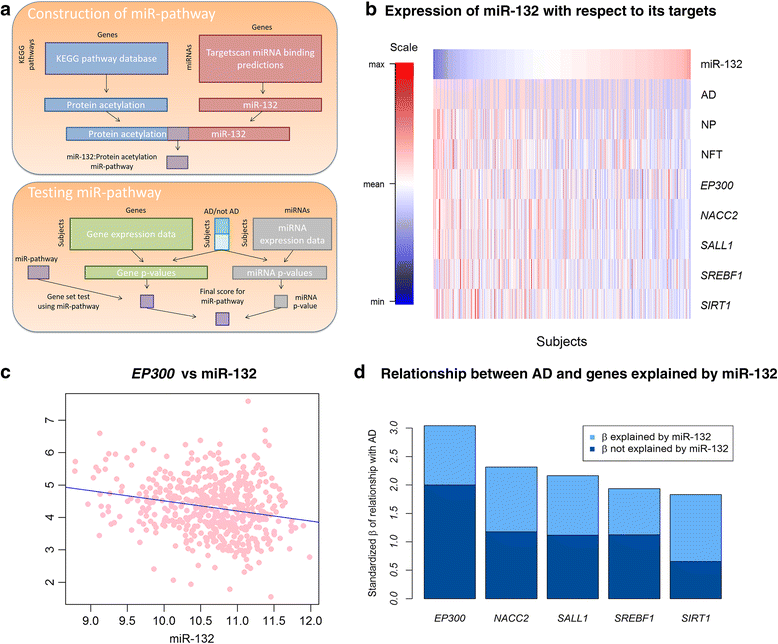
Results: We confirm the association of two well-established miRNA (miR-132, miR-129) with pathologic AD in our dataset and then further characterize this association in terms of its component neuritic β-amyloid plaques and neurofibrillary tangle pathologies. Additionally, we identify one new miRNA (miR-99) and four lincRNA that are associated with these traits. Many other previously reported associations of microRNA with AD are associated with the confounders quantified in our longitudinal cohort. Finally, by performing analyses integrating both miRNA and RNA sequence data from the same individuals (525 samples), we characterize the impact of AD associated miRNA on human brain expression: we show that the effects of miR-132 and miR-129-5b converge on certain genes such as EP300 and find a role for miR200 and its target genes in AD using an integrated miRNA/mRNA analysis.
Conclusions: Overall, miRNAs play a modest role in human AD, but we observe robust evidence that a small number of miRNAs are responsible for specific alterations in the cortical transcriptome that are associated with AD.
Keywords: Alzheimer’s disease; Neuritic β-amyloid plaques and neurofibrillary tangles; lincRNA; microRNA.
Conflict of interest statement
Ethics approval and consent to participate
The ROS and MAP studies were approved by the Institutional Review Board of Rush University Medical Center. All subjects have given written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- RF1 AG015819/AG/NIA NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- R01 AG032990/AG/NIA NIH HHS/United States
- RC2 AG036547/AG/NIA NIH HHS/United States
- U01 ES017155/ES/NIEHS NIH HHS/United States
- R01 AG018023/AG/NIA NIH HHS/United States
- RF1 AG036042/AG/NIA NIH HHS/United States
- U01 AG046152/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- K25 AG041906/AG/NIA NIH HHS/United States
- R01 AG036042/AG/NIA NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R01 AG036836/AG/NIA NIH HHS/United States
- R01 AG015819/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

