Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation
- PMID: 28669672
- PMCID: PMC5555377
- DOI: 10.1016/j.chom.2017.06.006
Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation
Abstract
Skin microbiota can impact allergic and autoimmune responses, wound healing, and anti-microbial defense. We investigated the role of skin microbiota in cutaneous leishmaniasis and found that human patients infected with Leishmania braziliensis develop dysbiotic skin microbiota, characterized by increases in the abundance of Staphylococcus and/or Streptococcus. Mice infected with L. major exhibit similar changes depending upon disease severity. Importantly, this dysbiosis is not limited to the lesion site, but is transmissible to normal skin distant from the infection site and to skin from co-housed naive mice. This observation allowed us to test whether a pre-existing dysbiotic skin microbiota influences disease, and we found that challenging dysbiotic naive mice with L. major or testing for contact hypersensitivity results in exacerbated skin inflammatory responses. These findings demonstrate that a dysbiotic skin microbiota is not only a consequence of tissue stress, but also enhances inflammation, which has implications for many inflammatory cutaneous diseases.
Keywords: cutaneous inflammation; dysbiosis; leishmania; microbiota; skin.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors list no conflicts of interest.
Figures


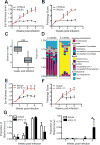
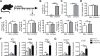



References
-
- Aguiar MG, Pereira AM, Fernandes AP, Ferreira LA. Reductions in skin and systemic parasite burdens as a combined effect of topical paromomycin and oral miltefosine treatment of mice experimentally infected with leishmania (leishmania) amazonensis. Antimicrob Agents Chemother. 2010;54:4699–4704. - PMC - PubMed
-
- Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol. Lett. 2005;101:226–230. - PubMed
-
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature. 2013;500:232–236. - PubMed
-
- Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, Gharbi A, Belhadj Hamida N, Boukthir A, Chlif S, et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 2013;368:524–532. - PubMed
-
- Canesso MC, Vieira AT, Castro TB, Schirmer BG, Cisalpino D, Martins FS, Rachid MA, Nicoli JR, Teixeira MM, Barcelos LS. Skin wound healing is accelerated and scarless in the absence of commensal microbiota. J. Immunol. 2014;193:5171–5180. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

