A Peptidergic Circuit Links the Circadian Clock to Locomotor Activity
- PMID: 28669757
- PMCID: PMC5698909
- DOI: 10.1016/j.cub.2017.05.089
A Peptidergic Circuit Links the Circadian Clock to Locomotor Activity
Abstract
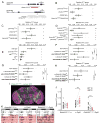
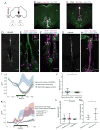
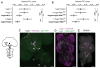
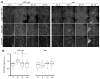
The mechanisms by which clock neurons in the Drosophila brain confer an ∼24-hr rhythm onto locomotor activity are unclear, but involve the neuropeptide diuretic hormone 44 (DH44), an ortholog of corticotropin-releasing factor. Here we identified DH44 receptor 1 as the relevant receptor for rest:activity rhythms and mapped its site of action to hugin-expressing neurons in the subesophageal zone (SEZ). We traced a circuit that extends from Dh44-expressing neurons in the pars intercerebralis (PI) through hugin+ SEZ neurons to the ventral nerve cord. Hugin neuropeptide, a neuromedin U ortholog, also regulates behavioral rhythms. The DH44 PI-Hugin SEZ circuit controls circadian locomotor activity in a daily cycle but has minimal effect on feeding rhythms, suggesting that the circadian drive to feed can be separated from circadian locomotion. These findings define a linear peptidergic circuit that links the clock to motor outputs to modulate circadian control of locomotor activity.
Keywords: DH44; Drosophila; Hugin; behavior; circadian rhythms; circuits; feeding; locomotion.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Hugin+ neurons provide a link between sleep homeostat and circadian clock neurons.Proc Natl Acad Sci U S A. 2021 Nov 23;118(47):e2111183118. doi: 10.1073/pnas.2111183118. Proc Natl Acad Sci U S A. 2021. PMID: 34782479 Free PMC article.
-
A circadian output center controlling feeding:fasting rhythms in Drosophila.PLoS Genet. 2019 Nov 6;15(11):e1008478. doi: 10.1371/journal.pgen.1008478. eCollection 2019 Nov. PLoS Genet. 2019. PMID: 31693685 Free PMC article.
-
Neuropeptide-Dependent Spike Time Precision and Plasticity in Circadian Output Neurons.Eur J Neurosci. 2025 Mar;61(5):e70037. doi: 10.1111/ejn.70037. Eur J Neurosci. 2025. PMID: 40080910 Free PMC article.
-
Molecular and circuit mechanisms mediating circadian clock output in the Drosophila brain.Eur J Neurosci. 2020 Jan;51(1):268-281. doi: 10.1111/ejn.14092. Epub 2018 Aug 16. Eur J Neurosci. 2020. PMID: 30059181 Free PMC article. Review.
-
Insect circadian clock outputs.Essays Biochem. 2011 Jun 30;49(1):87-101. doi: 10.1042/bse0490087. Essays Biochem. 2011. PMID: 21819386 Review.
Cited by
-
Drosophila Model for Studying Gut Microbiota in Behaviors and Neurodegenerative Diseases.Biomedicines. 2022 Mar 3;10(3):596. doi: 10.3390/biomedicines10030596. Biomedicines. 2022. PMID: 35327401 Free PMC article. Review.
-
Hugin+ neurons provide a link between sleep homeostat and circadian clock neurons.Proc Natl Acad Sci U S A. 2021 Nov 23;118(47):e2111183118. doi: 10.1073/pnas.2111183118. Proc Natl Acad Sci U S A. 2021. PMID: 34782479 Free PMC article.
-
Taste triggers a homeostatic temperature control in hungry flies.Elife. 2024 Dec 2;13:RP94703. doi: 10.7554/eLife.94703. Elife. 2024. PMID: 39621014 Free PMC article.
-
Cell type-specific driver lines targeting the Drosophila central complex and their use to investigate neuropeptide expression and sleep regulation.bioRxiv [Preprint]. 2025 Jan 24:2024.10.21.619448. doi: 10.1101/2024.10.21.619448. bioRxiv. 2025. Update in: Elife. 2025 Apr 17;14:RP104764. doi: 10.7554/eLife.104764. PMID: 39484527 Free PMC article. Updated. Preprint.
-
Differential gene expression in Drosophila melanogaster and D. nigrosparsa infected with the same Wolbachia strain.Sci Rep. 2021 May 31;11(1):11336. doi: 10.1038/s41598-021-90857-5. Sci Rep. 2021. PMID: 34059765 Free PMC article.
References
-
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. - PubMed
-
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

