An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period
- PMID: 28669797
- PMCID: PMC6348474
- DOI: 10.1016/j.bbi.2017.06.016
An examination of changes in maternal neuroimmune function during pregnancy and the postpartum period
Abstract
There is strong evidence that the immune system changes dramatically during pregnancy in order to prevent the developing fetus from being "attacked" by the maternal immune system. Due to these alterations in peripheral immune function, many women that suffer from autoimmune disorders actually find significant relief from their symptoms throughout pregnancy; however, these changes can also leave the mother more susceptible to infections that would otherwise be mitigated by the inflammatory response (Robinson and Klein, 2012). Only one other study has looked at changes in microglial number and morphology during pregnancy and the postpartum period (Haim et al., 2016), but no one has yet examined the neuroimmune response following an immune challenge during this time. Therefore, in this study, we investigated the impact of an immune challenge during various time-points throughout pregnancy and the postpartum period on the expression of immune molecules in the brain of the mother and fetus. Our results indicate that similar to the peripheral immune suppression measured during pregnancy, we also see significant suppression of the immune response in the maternal brain, particularly during late gestation. In contrast to the peripheral immune system, immune modulation in the maternal brain extends moderately into the postpartum period. Additionally, we found that the fetal immune response in the brain and placenta is also suppressed just before parturition, suggesting that cytokine production in the fetus and placenta are mirroring the peripheral cytokine response of the mother.
Keywords: Hormones; Immune function; Microglia; Mood and anxiety disorders; Peripartum depression; Pregnancy.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
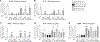
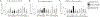
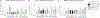
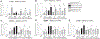
Similar articles
-
An investigation into the effects of antenatal stressors on the postpartum neuroimmune profile and depressive-like behaviors.Behav Brain Res. 2016 Feb 1;298(Pt B):218-28. doi: 10.1016/j.bbr.2015.11.011. Epub 2015 Nov 14. Behav Brain Res. 2016. PMID: 26589802 Free PMC article.
-
Maternal immune activation induced by lipopolysaccharide triggers immune response in pregnant mother and fetus, and induces behavioral impairment in adult rats.J Psychiatr Res. 2018 May;100:71-83. doi: 10.1016/j.jpsychires.2018.02.007. Epub 2018 Feb 10. J Psychiatr Res. 2018. PMID: 29494891
-
A survey of neuroimmune changes in pregnant and postpartum female rats.Brain Behav Immun. 2017 Jan;59:67-78. doi: 10.1016/j.bbi.2016.09.026. Epub 2016 Sep 26. Brain Behav Immun. 2017. PMID: 27686844
-
Microglia, the missing link in maternal immune activation and fetal neurodevelopment; and a possible link in preeclampsia and disturbed neurodevelopment?J Reprod Immunol. 2018 Apr;126:18-22. doi: 10.1016/j.jri.2018.01.004. Epub 2018 Jan 31. J Reprod Immunol. 2018. PMID: 29421625 Review.
-
Pregnancy Immunogenetics and Genomics: Implications for Pregnancy-Related Complications and Autoimmune Disease.Annu Rev Genomics Hum Genet. 2019 Aug 31;20:73-97. doi: 10.1146/annurev-genom-083118-014943. Epub 2019 Mar 8. Annu Rev Genomics Hum Genet. 2019. PMID: 30848957 Review.
Cited by
-
Cellular and molecular signatures of motherhood in the adult and ageing rat brain.Open Biol. 2023 Nov;13(11):230217. doi: 10.1098/rsob.230217. Epub 2023 Nov 22. Open Biol. 2023. PMID: 37989220 Free PMC article.
-
[Inflammatory Biomarkers and Postpartum Depression: A Systematic Review of Literature].Can J Psychiatry. 2019 Jul;64(7):471-481. doi: 10.1177/0706743719828970. Epub 2019 Feb 26. Can J Psychiatry. 2019. PMID: 30808206 Free PMC article. French.
-
Neuroimmunology of the female brain across the lifespan: Plasticity to psychopathology.Brain Behav Immun. 2019 Jul;79:39-55. doi: 10.1016/j.bbi.2019.03.010. Epub 2019 Mar 11. Brain Behav Immun. 2019. PMID: 30872093 Free PMC article. Review.
-
Chemical and non-chemical stressors in a postpartum cohort through wristband and self report data: Links between increased chemical burden, economic, and racial stress.Environ Int. 2024 Sep;191:108976. doi: 10.1016/j.envint.2024.108976. Epub 2024 Aug 23. Environ Int. 2024. PMID: 39216331 Free PMC article.
-
Animal models of congenital zika syndrome provide mechanistic insight into viral pathogenesis during pregnancy.PLoS Negl Trop Dis. 2020 Oct 22;14(10):e0008707. doi: 10.1371/journal.pntd.0008707. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33091001 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

