Morusin shows potent antitumor activity for human hepatocellular carcinoma in vitro and in vivo through apoptosis induction and angiogenesis inhibition
- PMID: 28670112
- PMCID: PMC5481341
- DOI: 10.2147/DDDT.S138320
Morusin shows potent antitumor activity for human hepatocellular carcinoma in vitro and in vivo through apoptosis induction and angiogenesis inhibition
Abstract
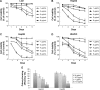
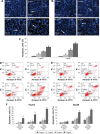
Hepatocellular carcinoma (HCC) is one of the most aggressive cancers with high mortality worldwide. Research and development of novel agents for HCC therapy is in demand, urgently. Morusin has been reported to exhibit potential cytotoxic activity in several cancer cell lines. However, whether it has potential antiangiogenic activity especially in HCC remains unclear. In the current study, we found that morusin exerted growth inhibition effects on human HCC cells (HepG2 and Hep3B) in vitro and human HCC cell (HepG2) xenografts in vivo. Moreover, apoptosis induction was observed in a dose-dependent manner after morusin treatment along with an increase in the expression of active caspase-3 and the Bax/Bcl-2 expression ratio. More importantly, morusin inhibited proliferation, migration, and tube formation of human umbilical vein endothelial cells (HUVECs) in vitro and downregulated angiogenic proteins in HCC cells and HUVECs. In vivo, tumor angiogenesis was also attenuated after morusin treatment. In addition, morusin suppressed constitutive as well as IL-6-induced STAT3 phosphorylation in HCC cells and corresponding tumor tissues. Overall, morusin has a potential anticancer effect on human HCC cells in vitro and in vivo by inducing apoptosis and inhibiting anti-angiogenesis. The corresponding mechanism might be associated with the attenuation of the IL-6/STAT3 signaling pathway. Morusin might serve as a promising novel anticancer agent in HCC therapy, and requires further study.
Keywords: IL-6; STAT3; angiogenesis; apoptosis; human hepatocellular carcinoma; morusin.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. - PubMed
-
- Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, Kamel IR, Ghasebeh MA, Pawlik TM. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25(2):74–85. - PubMed
-
- Connell LC, Harding JJ, Abou-Alfa GK. Advanced hepatocellular cancer: the current state of future research. Curr Treat Options Oncol. 2016;17(8):43. - PubMed
-
- Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol. 1997;8(2):117–136. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

