Sepsis-Associated Encephalopathy: The Blood-Brain Barrier and the Sphingolipid Rheostat
- PMID: 28670310
- PMCID: PMC5472697
- DOI: 10.3389/fimmu.2017.00597
Sepsis-Associated Encephalopathy: The Blood-Brain Barrier and the Sphingolipid Rheostat
Abstract
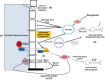
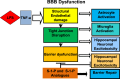
Sepsis is not only a significant cause of mortality worldwide but has particularly devastating effects on the central nervous system of survivors. It is therefore crucial to understand the molecular structure, physiology, and events involved in the pathogenesis of sepsis-associated encephalopathy, so that potential therapeutic advances can be achieved. A key determinant to the development of this type of encephalopathy is morphological and functional modification of the blood-brain barrier (BBB), whose function is to protect the CNS from pathogens and toxic threats. Key mediators of pathologic sequelae of sepsis in the brain include cytokines, including TNF-α, and sphingolipids, which are biologically active components of cellular membranes that possess diverse functions. Emerging data demonstrated an essential role for sphingolipids in the pulmonary vascular endothelium. This raises the question of whether endothelial stability in other organs systems such as the CNS may also be mediated by sphingolipids and their receptors. In this review, we will model the structure and vulnerability of the BBB and hypothesize mechanisms for therapeutic stabilization and repair following a confrontation with sepsis-induced inflammation.
Keywords: blood–brain barrier; inflammation mediators; lipopolysaccharides; sepsis-associated encephalopathy; sphingosine.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

