Neuroanatomy in mouse models of Rett syndrome is related to the severity of Mecp2 mutation and behavioral phenotypes
- PMID: 28670438
- PMCID: PMC5485541
- DOI: 10.1186/s13229-017-0138-8
Neuroanatomy in mouse models of Rett syndrome is related to the severity of Mecp2 mutation and behavioral phenotypes
Abstract
Background: Rett syndrome (RTT) is a neurodevelopmental disorder that predominantly affects girls. The majority of RTT cases are caused by de novo mutations in methyl-CpG-binding protein 2 (MECP2), and several mouse models have been created to further understand the disorder. In the current literature, many studies have focused their analyses on the behavioral abnormalities and cellular and molecular impairments that arise from Mecp2 mutations. However, limited efforts have been placed on understanding how Mecp2 mutations disrupt the neuroanatomy and networks of the brain.
Methods: In this study, we examined the neuroanatomy of male and female mice from the Mecp2tm1Hzo, Mecp2tm1.1Bird/J, and Mecp2tm2Bird/J mouse lines using high-resolution magnetic resonance imaging (MRI) paired with deformation-based morphometry to determine the brain regions susceptible to Mecp2 disruptions.
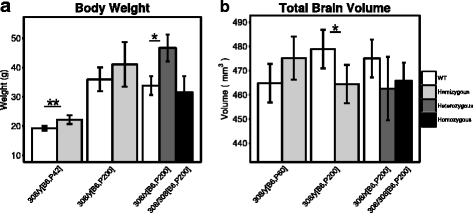
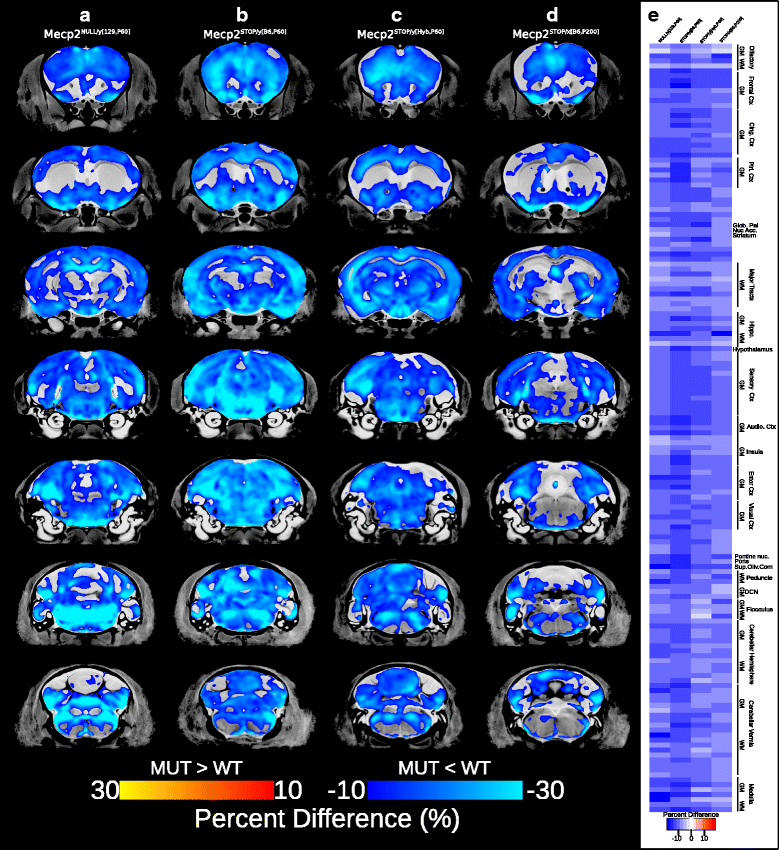
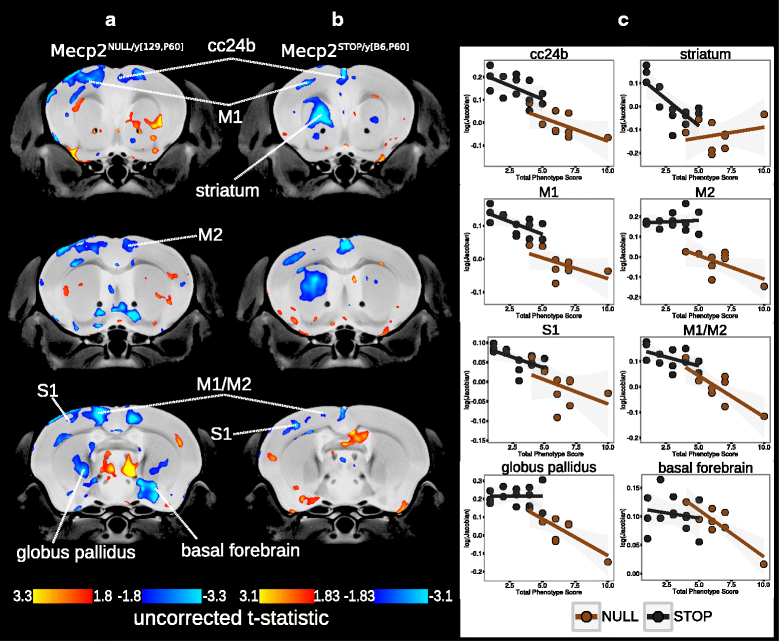
Results: We found that many cortical and subcortical regions were reduced in volume within the brains of mutant mice regardless of mutation type, highlighting regions that are susceptible to Mecp2 disruptions. We also found that the volume within these regions correlated with behavioral metrics. Conversely, regions of the cerebellum were differentially affected by the type of mutation, showing an increase in volume in the mutant Mecp2tm1Hzo brain relative to controls and a decrease in the Mecp2tm1.1Bird/J and Mecp2tm2Bird/J lines.
Conclusions: Our findings demonstrate that the direction and magnitude of the neuroanatomical differences between control and mutant mice carrying Mecp2 mutations are driven by the severity of the mutation and the stage of behavioral impairments.
Keywords: Magnetic resonance imaging; Mecp2 mouse models; Neuroanatomy; Rett syndrome.
Figures


References
-
- Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60(4):443–9. doi: 10.1203/01.pdr.0000238302.84552.d0. - DOI - PubMed
-
- Kaufmann W, Tierney E, Rohde C, Suarez-Pedraza M, Clarke M, Salorio C, Bibat G, Bukelis I, Naram D, Lanham D, et al. Social impairments in rett syndrome: characteristics and relationship with clinical severity. J Intellect Disabil Res. 2012;56(3):233–47. doi: 10.1111/j.1365-2788.2011.01404.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

