Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip
- PMID: 28670476
- PMCID: PMC5481871
- DOI: 10.4155/fsoa-2016-0091
Stem cell culture and differentiation in microfluidic devices toward organ-on-a-chip
Abstract
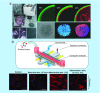
Microfluidic lab-on-a-chip provides a new platform with unique advantages to mimic complex physiological microenvironments in vivo and has been increasingly exploited to stem cell research. In this review, we highlight recent advances of microfluidic devices for stem cell culture and differentiation toward the development of organ-on-a-chip, especially with an emphasis on vital innovations within the last 2 years. Various aspects for improving on-chip stem-cell culture and differentiation, particularly toward organ-on-a-chip, are discussed, along with microenvironment control, surface modification, extracellular scaffolds, high throughput and stimuli. The combination of microfluidic technologies and stem cells hold great potential toward versatile systems of 'organ-on-a-chip' as desired. Adapted with permission from [1-8].
Keywords: microfluidic devices; organ-on-a-chip; stem cell; stem cell culture; stem cell differentiation.
Conflict of interest statement
Financial & competing interests disclosure The authors would like to acknowledge the financial support of the NIH/NIAID under award number R21AI107415 and the NIH/NIGMS under award number SC2GM105584. Financial support from the US NSF-PREM program (DMR 1205302), the IDR Program at the UTEP and the NIH RCMI Pilot Grant is also gratefully acknowledged. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures



References
-
- Matsumura T, Tatsumi K, Noda Y, et al. Single-cell cloning and expansion of human induced pluripotent stem cells by a microfluidic culture device. Biochem. Biophys. Res. Commun. 2014;453(1):131–137. - PubMed
-
- Jang JM, Tran SH, Na SC, Jeon NL. Engineering controllable architecture in matrigel for 3D cell alignment. ACS Appl. Mater. Interfaces. 2015;7(4):2183–2188. - PubMed
-
- Alessandri K, Feyeux M, Gurchenkov B, et al. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human neuronal stem cells (hNSC) Lab Chip. 2016;16(9):1593–1604. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
