PTEN is a negative regulator of mitotic checkpoint complex during the cell cycle
- PMID: 28670501
- PMCID: PMC5492438
- DOI: 10.1186/s40164-017-0079-0
PTEN is a negative regulator of mitotic checkpoint complex during the cell cycle
Abstract
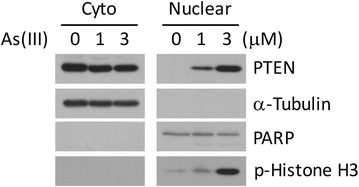
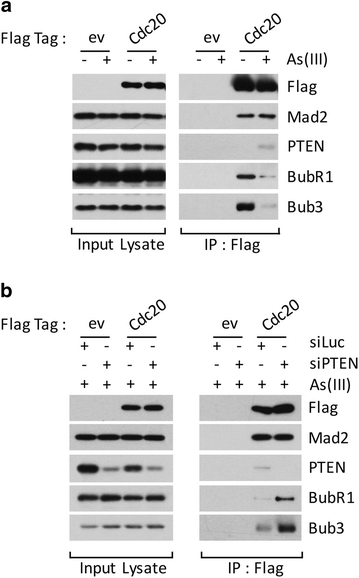
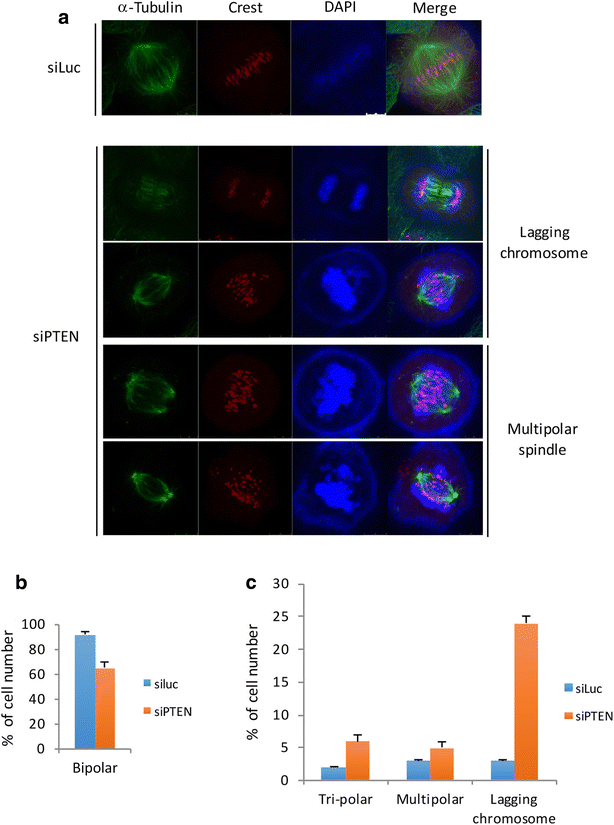
Nuclear PTEN plays an important role during mitosis. To understand the molecular basis by which PTEN mediates mitotic progression, we examined whether PTEN regulated the formation of mitotic checkpoint complex (MCC). We observed that arsenic trioxide, a mitotic inducer, stimulated nuclear translocation of PTEN in a time-dependent manner. PTEN physically interacted with Cdc20 and Mad2, two important components of MCC. Arsenic treatment diminished the physical association of PTEN with BubR1 and Bub3 but not with Cdc20 and Mad2. Our further studies revealed that downregulation of PTEN via RNAi enhanced formation of MCC during the cell cycle. Moreover, PTEN silencing induced chromosomal instability. Given the crucial role of PTEN in suppressing tumor development, our study strongly suggests that PTEN also functions to maintain chromosomal stability, partly through suppressing unscheduled formation of MCC.
Keywords: Chromosomes; Mitosis; Mitotic checkpoint complex; Nuclear localization; PTEN.
Figures

References
-
- Shen ZX, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89(9):3354–3360. - PubMed
-
- Chen GQ, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89(9):3345–3353. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

