Congenital Long QT syndrome and torsade de pointes
- PMID: 28670758
- PMCID: PMC6931590
- DOI: 10.1111/anec.12481
Congenital Long QT syndrome and torsade de pointes
Abstract
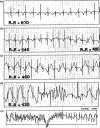
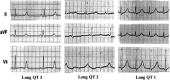
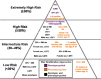
Since its initial description by Jervell and Lange-Nielsen in 1957, the congenital long QT syndrome (LQTS) has been the most investigated cardiac ion channelopathy. A prolonged QT interval in the surface electrocardiogram is the sine qua non of the LQTS and is a surrogate measure of the ventricular action potential duration (APD). Congenital as well as acquired alterations in certain cardiac ion channels can affect their currents in such a way as to increase the APD and hence the QT interval. The inhomogeneous lengthening of the APD across the ventricular wall results in dispersion of APD. This together with the tendency of prolonged APD to be associated with oscillations at the plateau level, termed early afterdepolarizations (EADs), provides the substrate of ventricular tachyarrhythmia associated with LQTS, usually referred to as torsade de pointes (TdP) VT. This review will discuss the genetic, molecular, and phenotype characteristics of congenital LQTS as well as current management strategies and future directions in the field.
Keywords: cellular electrophysiology; electrophsiology long QT syndrome; genetics; ion channels and membrane transporters; ventricular tachycardia.
© 2017 Wiley Periodicals, Inc.
Figures

References
-
- Andersen, E. D. , Krasilnikoff, P. A. , & Overvad, H. (1971). Intermittent muscular weakness, extrasystoles, and multiple developmental anomalies. A new syndrome? Acta Paediatrica Scandinavica, 60, 559–564. - PubMed
-
- Anderson, C. L. , Delisle, B. P. , Anson, B. D. , Kilby, J. A. , Will, M. L. , Tester, D. J. , … January, C. T. (2006). Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking‐deficient) mechanism. Circulation, 113(3), 365–373. - PubMed
-
- Antzelevitch, C. (2003). Molecular genetics of arrhythmias and cardiovascular conditions associated with arrhythmias. Journal of Cardiovascular Electrophysiology, 14, 1259–1272. - PubMed
-
- Arking, D. E. , Pfeufer, A. , Post, W. , Kao, W. H. , Newton‐Cheh, C. , Ikeda, M. , … West, K. (2006). A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nature Genetics, 38(6), 644–651. - PubMed
-
- Barhanin, J. , Lesage, F. , Guillemare, E. , Fink, M. , Lazdunski, M. , & Romey, G. (1996). K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature, 384(6604), 78–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

