Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism
- PMID: 28671190
- PMCID: PMC5500878
- DOI: 10.1038/ncomms15965
Compensatory metabolic networks in pancreatic cancers upon perturbation of glutamine metabolism
Abstract
Pancreatic ductal adenocarcinoma is a notoriously difficult-to-treat cancer and patients are in need of novel therapies. We have shown previously that these tumours have altered metabolic requirements, making them highly reliant on a number of adaptations including a non-canonical glutamine (Gln) metabolic pathway and that inhibition of downstream components of Gln metabolism leads to a decrease in tumour growth. Here we test whether recently developed inhibitors of glutaminase (GLS), which mediates an early step in Gln metabolism, represent a viable therapeutic strategy. We show that despite marked early effects on in vitro proliferation caused by GLS inhibition, pancreatic cancer cells have adaptive metabolic networks that sustain proliferation in vitro and in vivo. We use an integrated metabolomic and proteomic platform to understand this adaptive response and thereby design rational combinatorial approaches. We demonstrate that pancreatic cancer metabolism is adaptive and that targeting Gln metabolism in combination with these adaptive responses may yield clinical benefits for patients.
Conflict of interest statement
A.C.K. is a founder and has financial interests in Vescor Therapeutics, LLC. A.C.K. is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, targeting GOT1 as a therapeutic approach and the autophagic control of iron metabolism. A.C.K is on the SAB of Cornerstone Pharmaceuticals. J.D.M. is an inventor on a patent pertaining to the autophagic control of iron metabolism. All other authors declare no competing financial interests.
Figures

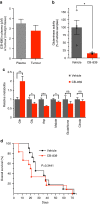



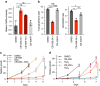
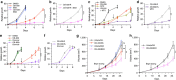

References
-
- Siegel R. L., Miller K. D. & Jemal A. Cancer statistics, 2016. CA. Cancer J. Clin. 66, 7–30 (2016). - PubMed
-
- Almoguera C. et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53, 549–554 (1988). - PubMed
-
- Bailey P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

