Superoxide Dismutase-Loaded Porous Polymersomes as Highly Efficient Antioxidants for Treating Neuropathic Pain
- PMID: 28671302
- PMCID: PMC5591629
- DOI: 10.1002/adhm.201700500
Superoxide Dismutase-Loaded Porous Polymersomes as Highly Efficient Antioxidants for Treating Neuropathic Pain
Abstract
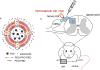
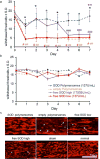
A highly efficient antioxidant is developed by encapsulating superoxide dismutase (SOD) within the aqueous interior of porous polymersomes. The porous polymersomes provide a permeable membrane that allows free superoxide radicals to pass into the aqueous interior and interact with the encapsulated antioxidant enzyme SOD. In vivo studies in the rat demonstrate that administration of SOD-encapsulated porous polymersomes can prevent neuropathic pain after nerve root compression more effectively than treatment with free antioxidant enzyme alone.
Keywords: antioxidants; neuropathic pain; porous polymersomes; superoxide dismutase.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

References
-
- Hogg-Johnson S, van der Velde G, Carroll LJ, Holm LW, Cassidy JD, Guzman J, Cote P, Haldeman S, Ammendolia C, Carragee E, Hurwitz E, Nordin M, Peloso P. Bone, P. Joint Decade - Task Force on Neck, D. Its Associated, Spine (Phila Pa 1976) 2008;33:S39. - PubMed
- Waters TR. J Electromyogr Kinesiol. 2004;14:7. - PubMed
- Cote P, Cassidy JD, Carroll LJ, Kristman V. Pain. 2004;112:267. - PubMed
-
- Scholz J, Woolf CJ. Nat Neurosci. 2007;10:1361. - PubMed
-
- Radhakrishnan K, Litchy WJ, O'Fallon WM, Kurland LT. Brain. 1994;117(Pt 2):325. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

