Influence of Environmental Factors on the Production of Penitrems A-F by Penicillium crustosum
- PMID: 28671569
- PMCID: PMC5535157
- DOI: 10.3390/toxins9070210
Influence of Environmental Factors on the Production of Penitrems A-F by Penicillium crustosum
Abstract
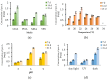
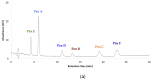
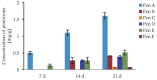
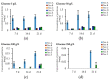
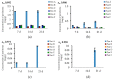
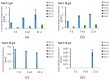
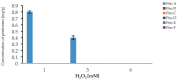
Filamentous fungi produce a multitude of secondary metabolites, some of them known as mycotoxins, which are toxic to vertebrates and other animal groups in low concentrations. Among them, penitrems, which belong to the group of indole-diterpene mycotoxins, are synthesized by Penicillium and Aspergillus genera and exhibit potent tremorgenic effects. This is the first complex study of the penitrems A-F production under the influence of different abiotic factors, e.g., media, incubation time, temperature, pH, light, water activity, and carbon and nitrogen source as well as oxidative and salt stress. For this purpose, penitrems A-F were isolated from Penicillium crustosum cultures and used as analytical standards. Among the carbon sources, glucose supplemented to the media at the concentration of 50 g/L, showed the strongest inducing effect on the biosynthesis of penitrems. Among nitrogen sources, glutamate was found to be the most favorable supplement, significantly increasing production of these secondary metabolites. CuSO4-promoted oxidative stress was also shown to remarkably stimulate biosynthesis of all penitrems. In contrast, the salt stress, caused by the elevated concentrations of NaCl, showed an inhibitory effect on the penitrem biosynthesis. Finally, cheese model medium elicited exceptionally high production of all members of the penitrems family. Obtained results give insides into the biosynthesis of toxicologically relevant penitrems A-F under different environmental factors and can be utilized to prevent food contamination.
Keywords: Penicillium crustosum; cheese model medium; flash chromatography; liquid chromatography; micro-scale extraction; penitrem; stress factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Detection of the Cytotoxic Penitrems A-F in Cheese from the European Single Market by HPLC-MS/MS.J Agric Food Chem. 2018 Feb 7;66(5):1264-1269. doi: 10.1021/acs.jafc.7b06001. Epub 2018 Jan 25. J Agric Food Chem. 2018. PMID: 29338236
-
Biosynthesis of penitrems and roquefortine by Penicillium crustosum.Appl Environ Microbiol. 1983 May;45(5):1486-90. doi: 10.1128/aem.45.5.1486-1490.1983. Appl Environ Microbiol. 1983. PMID: 6870239 Free PMC article.
-
Penitrem and thomitrem formation by Penicillium crustosum.Mycopathologia. 2004 Apr;157(3):349-57. doi: 10.1023/b:myco.0000024180.99262.b1. Mycopathologia. 2004. PMID: 15180164
-
[Nitrogen-containing mycotoxins of fungi of Aspergillus and Penicillium species infesting grain and its products].Prikl Biokhim Mikrobiol. 1993 Nov-Dec;29(6):814-22. Prikl Biokhim Mikrobiol. 1993. PMID: 8295871 Review. Russian.
-
PCR-based diagnosis and quantification of mycotoxin producing fungi.Int J Food Microbiol. 2007 Oct 20;119(1-2):38-46. doi: 10.1016/j.ijfoodmicro.2007.07.023. Epub 2007 Jul 31. Int J Food Microbiol. 2007. PMID: 17804102 Review.
Cited by
-
The hidden power of secondary metabolites in plant-fungi interactions and sustainable phytoremediation.Front Plant Sci. 2022 Dec 12;13:1044896. doi: 10.3389/fpls.2022.1044896. eCollection 2022. Front Plant Sci. 2022. PMID: 36578344 Free PMC article. Review.
-
Construction of an expression platform for fungal secondary metabolite biosynthesis in Penicillium crustosum.Appl Microbiol Biotechnol. 2024 Jul 24;108(1):427. doi: 10.1007/s00253-024-13259-3. Appl Microbiol Biotechnol. 2024. PMID: 39046587 Free PMC article.
-
Tremorgenic and neurotoxic paspaline-derived indole-diterpenes: biosynthetic diversity, threats and applications.Appl Microbiol Biotechnol. 2019 Feb;103(4):1599-1616. doi: 10.1007/s00253-018-09594-x. Epub 2019 Jan 6. Appl Microbiol Biotechnol. 2019. PMID: 30613899 Free PMC article. Review.
-
Molecular regulation of fungal secondary metabolism.World J Microbiol Biotechnol. 2023 May 20;39(8):204. doi: 10.1007/s11274-023-03649-6. World J Microbiol Biotechnol. 2023. PMID: 37209190 Review.
-
Fungal secondary metabolism: regulation, function and drug discovery.Nat Rev Microbiol. 2019 Mar;17(3):167-180. doi: 10.1038/s41579-018-0121-1. Nat Rev Microbiol. 2019. PMID: 30531948 Free PMC article. Review.
References
-
- Steyn P.S., Vleggaar R. Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Springer; Vienna, Austria: 1985. Tremorgenic mycotoxins; pp. 1–80. - PubMed
-
- Smith M.M., Warren V.A., Thomas B.S., Brochu R.M., Ertel E.A., Rohrer S., Schaeffer J., Schmatz D., Petuch B.R., Tang Y.S., et al. Nodulisporic acid opens insect glutamate-gated chloride channels: Identification of a new high affinity modulator. Biochemistry. 2000;39:5543–5554. doi: 10.1021/bi992943i. - DOI - PubMed
-
- Ondeyka J.G., Helms G.L., Hensens O.D., Goetz M.A., Zink D.L., Tsipouras A., Shoop W.L., Slayton L., Dombrowski A.W., Polishook J.D., et al. Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp. Isolation, structure determination, and chemical transformations. J. Am. Chem. Soc. 1997;119:8809–8816. doi: 10.1021/ja971664k. - DOI
-
- De Jesus A.E., Gorst-Allman C.P., Steyn P.S., van Heerden F.R., Vleggaar R., Wessels P.L., Hull W.E. Tremorgenic mycotoxins from Penicillium crustosum Biosynthesis of Penitrem A. J. Chem. Soc. Perkin Trans. 1. 1983;8:1863–1868. doi: 10.1039/p19830001863. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

