Lipotoxicity-Induced PRMT1 Exacerbates Mesangial Cell Apoptosis via Endoplasmic Reticulum Stress
- PMID: 28671608
- PMCID: PMC5535913
- DOI: 10.3390/ijms18071421
Lipotoxicity-Induced PRMT1 Exacerbates Mesangial Cell Apoptosis via Endoplasmic Reticulum Stress
Abstract
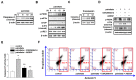
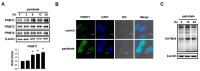
Lipotoxicity-induced mesangial cell apoptosis is implicated in the exacerbation of diabetic nephropathy (DN). Protein arginine methyltransferases (PRMTs) have been known to regulate a variety of biological functions. Recently, it was reported that PRMT1 expression is increased in proximal tubule cells under diabetic conditions. However, their roles in mesangial cells remain unexplored. Thus, we examined the pathophysiological roles of PRMTs in mesangial cell apoptosis. Treatment with palmitate, which mimics cellular lipotoxicity, induced mesangial cell apoptosis via protein kinase RNA-like endoplasmic reticulum kinase (PERK) and ATF6-mediated endoplasmic reticulum (ER) stress signaling. Palmitate treatment increased PRMT1 expression and activity in mesangial cells as well. Moreover, palmitate-induced ER stress activation and mesangial cell apoptosis was diminished by PRMT1 knockdown. In the mice study, high fat diet-induced glomerular apoptosis was attenuated in PRMT1 haploinsufficient mice. Together, these results provide evidence that lipotoxicity-induced PRMT1 expression promotes ER stress-mediated mesangial cell apoptosis. Strategies to regulate PRMT1 expression or activity could be used to prevent the exacerbation of DN.
Keywords: ER stress; PRMT1; apoptosis; lipotoxicity; mesangial cell.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Protein arginine methyltranferase-1 induces ER stress and epithelial-mesenchymal transition in renal tubular epithelial cells and contributes to diabetic nephropathy.Biochim Biophys Acta Mol Basis Dis. 2019 Oct 1;1865(10):2563-2575. doi: 10.1016/j.bbadis.2019.06.001. Epub 2019 Jun 11. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 31199999
-
Asymmetric dimethylarginine (ADMA) treatment induces apoptosis in cultured rat mesangial cells via endoplasmic reticulum stress activation.Cell Biol Int. 2016 Jun;40(6):662-70. doi: 10.1002/cbin.10602. Epub 2016 Apr 6. Cell Biol Int. 2016. PMID: 26992443
-
Fatty Acid-Binding Protein 4 mediates apoptosis via endoplasmic reticulum stress in mesangial cells of diabetic nephropathy.Mol Cell Endocrinol. 2015 Aug 15;411:232-42. doi: 10.1016/j.mce.2015.05.003. Epub 2015 May 6. Mol Cell Endocrinol. 2015. PMID: 25958041
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
The Role of Endoplasmic Reticulum Stress in Diabetic Nephropathy.Curr Diab Rep. 2017 Mar;17(3):17. doi: 10.1007/s11892-017-0842-y. Curr Diab Rep. 2017. PMID: 28271468 Review.
Cited by
-
Insulin Resistance, Obesity, and Lipotoxicity.Adv Exp Med Biol. 2024;1460:391-430. doi: 10.1007/978-3-031-63657-8_14. Adv Exp Med Biol. 2024. PMID: 39287860 Review.
-
Protein arginine methyltransferases in renal development, injury, repair, and fibrosis.Front Pharmacol. 2023 Feb 3;14:1123415. doi: 10.3389/fphar.2023.1123415. eCollection 2023. Front Pharmacol. 2023. PMID: 36817133 Free PMC article. Review.
-
Biomarkers of Arginine Methylation in Diabetic Nephropathy: Novel Insights from Bioinformatics Analysis.Diabetes Metab Syndr Obes. 2024 Sep 13;17:3399-3418. doi: 10.2147/DMSO.S472412. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39290792 Free PMC article.
-
Homocysteine induces human mesangial cell apoptosis via the involvement of autophagy and endoplasmic reticulum stress.RSC Adv. 2019 Oct 7;9(54):31720-31727. doi: 10.1039/c9ra04248b. eCollection 2019 Oct 1. RSC Adv. 2019. PMID: 35527928 Free PMC article.
-
Signaling pathways of chronic kidney diseases, implications for therapeutics.Signal Transduct Target Ther. 2022 Jun 9;7(1):182. doi: 10.1038/s41392-022-01036-5. Signal Transduct Target Ther. 2022. PMID: 35680856 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

