Vascular Mechanobiology: Towards Control of In Situ Regeneration
- PMID: 28671618
- PMCID: PMC5617965
- DOI: 10.3390/cells6030019
Vascular Mechanobiology: Towards Control of In Situ Regeneration
Abstract
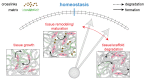
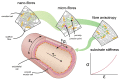
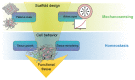
The paradigm of regenerative medicine has recently shifted from in vitro to in situ tissue engineering: implanting a cell-free, biodegradable, off-the-shelf available scaffold and inducing the development of functional tissue by utilizing the regenerative potential of the body itself. This approach offers a prospect of not only alleviating the clinical demand for autologous vessels but also circumventing the current challenges with synthetic grafts. In order to move towards a hypothesis-driven engineering approach, we review three crucial aspects that need to be taken into account when regenerating vessels: (1) the structure-function relation for attaining mechanical homeostasis of vascular tissues, (2) the environmental cues governing cell function, and (3) the available experimental platforms to test instructive scaffolds for in situ tissue engineering. The understanding of cellular responses to environmental cues leads to the development of computational models to predict tissue formation and maturation, which are validated using experimental platforms recapitulating the (patho)physiological micro-environment. With the current advances, a progressive shift is anticipated towards a rational and effective approach of building instructive scaffolds for in situ vascular tissue regeneration.
Keywords: growth and remodeling; in situ tissue engineering; mechanosensing; mechanotransduction; regeneration; scaffolds; tissue homeostasis; vessels.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Biomaterial-driven in situ cardiovascular tissue engineering-a multi-disciplinary perspective.NPJ Regen Med. 2017 Jun 16;2:18. doi: 10.1038/s41536-017-0023-2. eCollection 2017. NPJ Regen Med. 2017. PMID: 29302354 Free PMC article. Review.
-
Inconsistency in Graft Outcome of Bilayered Bioresorbable Supramolecular Arterial Scaffolds in Rats.Tissue Eng Part A. 2021 Jul;27(13-14):894-904. doi: 10.1089/ten.TEA.2020.0185. Epub 2020 Oct 5. Tissue Eng Part A. 2021. PMID: 32873211
-
A mesofluidics-based test platform for systematic development of scaffolds for in situ cardiovascular tissue engineering.Tissue Eng Part C Methods. 2012 Jun;18(6):475-85. doi: 10.1089/ten.TEC.2011.0458. Epub 2012 Feb 10. Tissue Eng Part C Methods. 2012. PMID: 22224590
-
Inflammatory and regenerative processes in bioresorbable synthetic pulmonary valves up to two years in sheep-Spatiotemporal insights augmented by Raman microspectroscopy.Acta Biomater. 2021 Nov;135:243-259. doi: 10.1016/j.actbio.2021.09.005. Epub 2021 Sep 10. Acta Biomater. 2021. PMID: 34509697
-
Biomaterials for In Situ Tissue Regeneration: A Review.Biomolecules. 2019 Nov 19;9(11):750. doi: 10.3390/biom9110750. Biomolecules. 2019. PMID: 31752393 Free PMC article. Review.
Cited by
-
Topography-driven alterations in endothelial cell phenotype and contact guidance.Heliyon. 2020 Jun 29;6(6):e04329. doi: 10.1016/j.heliyon.2020.e04329. eCollection 2020 Jun. Heliyon. 2020. PMID: 32637708 Free PMC article.
-
A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions.Lab Chip. 2018 May 29;18(11):1607-1620. doi: 10.1039/c8lc00286j. Lab Chip. 2018. PMID: 29756630 Free PMC article.
-
Biomimetic Glycosaminoglycan-Analog Hydrogel for Improved Embolization of Aneurysms: Environment-Selective Swelling.Adv Healthc Mater. 2025 Jul;14(17):e2404506. doi: 10.1002/adhm.202404506. Epub 2025 Apr 7. Adv Healthc Mater. 2025. PMID: 40192445 Free PMC article.
-
An Investigation of the Constructional Design Components Affecting the Mechanical Response and Cellular Activity of Electrospun Vascular Grafts.Membranes (Basel). 2022 Sep 25;12(10):929. doi: 10.3390/membranes12100929. Membranes (Basel). 2022. PMID: 36295688 Free PMC article. Review.
-
Cellular Geometry Sensing at Different Length Scales and its Implications for Scaffold Design.Materials (Basel). 2020 Feb 21;13(4):963. doi: 10.3390/ma13040963. Materials (Basel). 2020. PMID: 32098110 Free PMC article. Review.
References
-
- Putnam A.J. Mechanobiological control of cell fate for applications in cardiovascular regenerative medicine. In: Chien S., Engler A.J., Wang P.Y., editors. Molecular and Cellular Mechanobiology. Springer; New York, NY, USA: 2016. pp. 219–253.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

