Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-κB Signaling
- PMID: 28671640
- PMCID: PMC5535915
- DOI: 10.3390/ijms18071424
Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-κB Signaling
Abstract
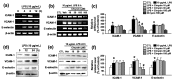
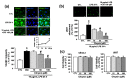
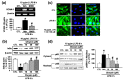
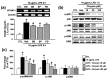
Adhesion of leukocytes to endothelial cells plays an important role in neuroinflammation. Therefore, suppression of the expression of adhesion molecules in brain endothelial cells may inhibit neuroinflammation. Chrysin (5,7-dihydroxyflavone) is a flavonoid component of propolis, blue passion flowers, and fruits. In the present study, we examined the effects of chrysin on lipopolysaccharide (LPS)-induced expression of vascular cell adhesion molecule-1 (VCAM-1) in mouse cerebral vascular endothelial (bEnd.3) cells. In bEnd.3 cells, LPS increased mRNA expression of VCAM-1 in a time-dependent manner, and chrysin significantly decreased LPS-induced mRNA expression of VCAM-1. Chrysin also reduced VCAM-1 protein expression in a concentration-dependent manner. Furthermore, chrysin blocked adhesion of monocytes to bEnd.3 cells exposed to LPS. Nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase, which are all activated by LPS, were significantly inhibited by chrysin. These results indicate that chrysin inhibits the expression of VCAM-1 in brain endothelial cells by inhibiting NF-κB translocation and MAPK signaling, resulting in the attenuation of leukocyte adhesion to endothelial cells. The anti-inflammatory effects of chrysin suggest a possible therapeutic application of this agent to neurodegenerative diseases, such as multiple sclerosis, septic encephalopathy, and allergic encephalomyelitis.
Keywords: blood-brain barrier; brain endothelial cell; chrysin; lipopolysaccharide; monocyte adhesion; neuroinflammation; vascular cell adhesion molecule-1 (VCAM-1).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Involvement of MAPKs and NF-kappaB in LPS-induced VCAM-1 expression in human tracheal smooth muscle cells.Cell Signal. 2007 Jun;19(6):1258-67. doi: 10.1016/j.cellsig.2007.01.009. Epub 2007 Jan 19. Cell Signal. 2007. PMID: 17303384
-
Visfatin Promotes Monocyte Adhesion by Upregulating ICAM-1 and VCAM-1 Expression in Endothelial Cells via Activation of p38-PI3K-Akt Signaling and Subsequent ROS Production and IKK/NF-κB Activation.Cell Physiol Biochem. 2019;52(6):1398-1411. doi: 10.33594/000000098. Cell Physiol Biochem. 2019. PMID: 31075190
-
Vascular cell adhesion molecule-1 expression in human intestinal microvascular endothelial cells is regulated by PI 3-kinase/Akt/MAPK/NF-kappaB: inhibitory role of curcumin.Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G259-68. doi: 10.1152/ajpgi.00087.2009. Epub 2009 Jun 11. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19520742 Free PMC article.
-
Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants.Antioxid Redox Signal. 2011 Sep 15;15(6):1607-38. doi: 10.1089/ars.2010.3522. Epub 2011 May 11. Antioxid Redox Signal. 2011. PMID: 21050132 Free PMC article. Review.
-
Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation.FASEB J. 2018 Aug;32(8):4070-4084. doi: 10.1096/fj.201701536R. Epub 2018 Mar 22. FASEB J. 2018. PMID: 29565737 Review.
Cited by
-
Metabolic Endotoxemia: From the Gut to Neurodegeneration.Int J Mol Sci. 2024 Jun 26;25(13):7006. doi: 10.3390/ijms25137006. Int J Mol Sci. 2024. PMID: 39000116 Free PMC article. Review.
-
Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood-Brain Barrier in Diseases.Nutrients. 2025 Feb 21;17(5):766. doi: 10.3390/nu17050766. Nutrients. 2025. PMID: 40077636 Free PMC article. Review.
-
Heme oxygenase-1 modulates CD62E-dependent endothelial cell-monocyte interactions and mitigates HLA-I-induced transplant vasculopathy in mice.Front Immunol. 2025 Mar 7;16:1447319. doi: 10.3389/fimmu.2025.1447319. eCollection 2025. Front Immunol. 2025. PMID: 40124367 Free PMC article.
-
The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion.Int J Mol Sci. 2020 Apr 25;21(9):3031. doi: 10.3390/ijms21093031. Int J Mol Sci. 2020. PMID: 32344794 Free PMC article. Review.
-
Anti-Inflammatory Potential of 3-Hydroxy-β-Ionone from Moringa oleifera: Decreased Transendothelial Migration of Monocytes Through an Inflamed Human Endothelial Cell Monolayer by Inhibiting the IκB-α/NF-κB Signaling Pathway.Molecules. 2024 Dec 12;29(24):5873. doi: 10.3390/molecules29245873. Molecules. 2024. PMID: 39769962 Free PMC article.
References
-
- Coles A.J., Compston D.A., Selmaj K.W., Lake S.L., Moran S., Margolin D.H., Norris K., Tandon P.K. Alemtuzumab vs. interferon β-1a in early multiple sclerosis. N. Engl. J. Med. 2008;359:1786–1801. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

