Monitoring thioredoxin redox with a genetically encoded red fluorescent biosensor
- PMID: 28671680
- PMCID: PMC5605834
- DOI: 10.1038/nchembio.2417
Monitoring thioredoxin redox with a genetically encoded red fluorescent biosensor
Abstract
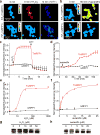
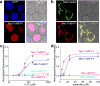
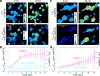
Thioredoxin (Trx) is one of the two major thiol antioxidants, playing essential roles in redox homeostasis and signaling. Despite its importance, there is a lack of methods for monitoring Trx redox dynamics in live cells, hindering a better understanding of physiological and pathological roles of the Trx redox system. In this work, we developed the first genetically encoded fluorescent biosensor for Trx redox by engineering a redox relay between the active-site cysteines of human Trx1 and rxRFP1, a redox-sensitive red fluorescent protein. We used the resultant biosensor-TrxRFP1-to selectively monitor perturbations of Trx redox in various mammalian cell lines. We subcellularly localized TrxRFP1 to image compartmentalized Trx redox changes. We further combined TrxRFP1 with a green fluorescent Grx1-roGFP2 biosensor to simultaneously monitor Trx and glutathione redox dynamics in live cells in response to chemical and physiologically relevant stimuli.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014;66:75–87. - PubMed
-
- Wei SJ, et al. Thioredoxin nuclear translocation and interaction with redox factor-1 activates the activator protein-1 transcription factor in response to ionizing radiation. Cancer Res. 2000;60:6688–95. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

