Reorganization of corticospinal output during motor learning
- PMID: 28671694
- PMCID: PMC5656286
- DOI: 10.1038/nn.4596
Reorganization of corticospinal output during motor learning
Abstract
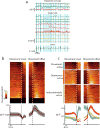
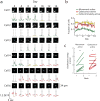
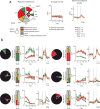
Motor learning is accompanied by widespread changes within the motor cortex, but it is unknown whether these changes are ultimately funneled through a stable corticospinal output channel or whether the corticospinal output itself is plastic. We investigated the consistency of the relationship between corticospinal neuron activity and movement through in vivo two-photon calcium imaging in mice learning a lever-press task. Corticospinal neurons exhibited heterogeneous correlations with movement, with the majority of movement-modulated neurons decreasing activity during movement. Individual cells changed their activity across days, which led to changed associations between corticospinal activity and movement. Unlike previous observations in layer 2/3, activity accompanying learned movements did not become more consistent with learning; instead, the activity of dissimilar movements became more decorrelated. These results indicate that the relationship between corticospinal activity and movement is dynamic and that the types of activity and plasticity are different from and possibly complementary to those in layer 2/3.
Figures




References
-
- Heffner R, Masterton B. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain Behav Evol. 1975;12:161–200. - PubMed
-
- Armand J. The origin, course and terminations of corticospinal fibers in various mammals. Prog Brain Res. 1982;57:329–60. - PubMed
-
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. - PubMed
-
- Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci. 2009;12:1586–93. - PubMed

