Glycaemic outcomes of an Individualized treatMent aPproach for oldER vulnerable patIents: A randomized, controlled stUdy in type 2 diabetes Mellitus (IMPERIUM)
- PMID: 28671753
- PMCID: PMC5724506
- DOI: 10.1111/dom.13051
Glycaemic outcomes of an Individualized treatMent aPproach for oldER vulnerable patIents: A randomized, controlled stUdy in type 2 diabetes Mellitus (IMPERIUM)
Abstract
Aims: To compare the glycaemic outcomes of 2 glucose-lowering treatment strategies in vulnerable (moderately ill and/or frail) patients aged ≥65 years with type 2 diabetes whose individual HbA1c targets were not met with diet/exercise and/or oral anti-hyperglycaemic medications (OAMs).
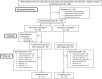
Methods: The primary endpoint of this study was a composite of achieving/maintaining individualized HbA1c targets without "clinically significant" hypoglycaemia (severe hypoglycaemia or repeated hypoglycaemia causing interruption of patients' activities or blood glucose <54 mg/dL). Strategy-A comprised glucose-dependent therapies (n = 99) with a non-sulphonylurea OAM and a glucagon-like peptide-1 receptor agonist as the first injectable. Strategy-B comprised non-glucose-dependent therapies (n = 93) with sulphonylurea as the preferred OAM and insulin glargine as the first injectable.
Results: There was no significant difference between Strategy-A and Strategy-B in percentages of patients achieving the primary endpoint (64.5% vs 54.9%; P = .190). Mean incidences (A vs B) of total (10.2% vs 53.8%), documented symptomatic (5.1% vs 36.6%), and asymptomatic (8.2% vs 32.3%) hypoglycaemia were lower for Strategy-A (P < .001 each). Proportions of patients achieving/maintaining HbA1c target (A, 63.3% vs B, 55.9%) were similar.
Conclusion: Similar proportions of older, vulnerable aged ≥65 years patients with type 2 diabetes achieved/maintained glycaemic treatment goals without clinically significant hypoglycaemia with Strategies A or B. However, Strategy-A resulted in lower risk of total, documented symptomatic, and asymptomatic hypoglycaemia. These results identify an approach of potential clinical benefit in this age group and will inform future clinical research in older patients with type 2 diabetes.
Trial registration: ClinicalTrials.gov NCT02072096.
Keywords: GLP-1 analogue; glycaemic control; hypoglycaemia; insulin delivery; sulphonylureas.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
S. R. H. has undertaken consultancy for Novo‐Nordisk, Eli Lilly and Company, Sanofi, Boehringer‐Ingelheim, Merck Sharp & Dohme, and Takeda. He has received research support from Medtronic and has been a member of speaker bureaus for Novo‐Nordisk, Eli Lilly and Company, Sanofi, Takeda, and Merck Sharp & Dohme.
R. E. P. consulted for AstraZeneca, Boehringer‐Ingelheim, GlaxoSmithKline, Hanmi Pharmaceutical Co., Janssen Pharmaceuticals, Ligand Pharmaceuticals, Eli Lilly and Company, Merck, Novo‐Nordisk and Takeda. He has received research support from Eli Lilly and Company, Merck Sharp & Dohme, Novo‐Nordisk, Sanofi, and Takeda and has spoken on behalf of AstraZeneca and Novo‐Nordisk. All honoraria and fees from these activities have been directed to a non‐profit. R. E. P. does not receive any direct or indirect compensation for these services. A.S. has received consultancy fees from Merck, Takeda, Novartis and Eli Lilly and Company. A. F., J. K., C. S. B., R. D. and R. J. H. are full‐time employees and minor stockholders of Eli Lilly and Company.
Figures
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 7th ed. 2015. http://www.diabetesatlas.org/. Accessed October 14, 2016.
-
- Gambert SR, Pinkstaff S. Emerging epidemic: diabetes in older adults: demography, economic impact, and pathophysiology. Diabetes Spectrum. 2006;19:221.
-
- Sloan FA, Bethel MA, Ruiz D Jr, Shea AM, Feinglos MN. The growing burden of diabetes mellitus in the US elderly population. Arch Intern Med. 2008;168:192–199. - PubMed
-
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. - PubMed
-
- Sinclair A, Morley J. Frailty and diabetes. Lancet. 2013;382:1386–1387. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous