Efficient Reduction of Vertebrate Cytoglobins by the Cytochrome b5/Cytochrome b5 Reductase/NADH System
- PMID: 28671819
- PMCID: PMC5555378
- DOI: 10.1021/acs.biochem.7b00224
Efficient Reduction of Vertebrate Cytoglobins by the Cytochrome b5/Cytochrome b5 Reductase/NADH System
Abstract
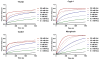
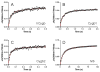
Cytoglobin is a heme-containing protein ubiquitous in mammalian tissues. Unlike the evolutionarily related proteins hemoglobin and myoglobin, cytoglobin shows a six-coordinated heme binding, with the heme iron coordinated by two histidine side chains. Cytoglobin is involved in cytoprotection pathways through yet undefined mechanisms, and it has recently been demonstrated that cytoglobin has redox signaling properties via nitric oxide (NO) and nitrite metabolism. The reduced, ferrous cytoglobin can bind oxygen and will react with NO in a dioxygenation reaction to form nitrate, which dampens NO signaling. When deoxygenated, cytoglobin can bind nitrite and reduce it to NO. This oxidoreductase activity could be catalytic if an effective reduction system exists to regenerate the reduced heme species. The nature of the physiological cytoglobin reducing system is unknown, although it has been proposed that ascorbate and cytochrome b5 could fulfill this role. Here we describe that physiological concentrations of cytochrome b5 and cytochrome b5 reductase can reduce human and fish cytoglobins at rates up to 250-fold higher than those reported for their known physiological substrates, hemoglobin and myoglobin, and up to 100-fold faster than 5 mM ascorbate. These data suggest that the cytochrome b5/cytochrome b5 reductase system is a viable reductant for cytoglobin in vivo, allowing for catalytic oxidoreductase activity.
Figures






References
-
- Kawada N, Kristensen DB, Asahina K, Nakatani K, Minamiyama Y, Seki S, Yoshizato K. Characterization of a stellate cell activation-associated protein (STAP) with peroxidase activity found in rat hepatic stellate cells. J Biol Chem. 2001;276:25318–25323. - PubMed
-
- Burmester T, Ebner B, Weich B, Hankeln T. Cytoglobin: a novel globin type ubiquitously expressed in vertebrate tissues. Mol Biol Evol. 2002;19:416–421. - PubMed
-
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. - PubMed
-
- Trent JT, 3rd, Hargrove MS. A ubiquitously expressed human hexacoordinate hemoglobin. J Biol Chem. 2002;277:19538–19545. - PubMed
-
- Hankeln T, Wystub S, Laufs T, Schmidt M, Gerlach F, Saaler-Reinhardt S, Reuss S, Burmester T. The cellular and subcellular localization of neuroglobin and cytoglobin -- a clue to their function? IUBMB Life. 2004;56:671–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

