Checkpoints of apicomplexan cell division identified in Toxoplasma gondii
- PMID: 28671988
- PMCID: PMC5510908
- DOI: 10.1371/journal.ppat.1006483
Checkpoints of apicomplexan cell division identified in Toxoplasma gondii
Abstract
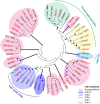
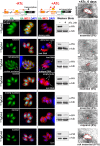
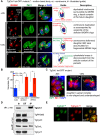
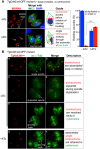
The unusual cell cycles of Apicomplexa parasites are remarkably flexible with the ability to complete cytokinesis and karyokinesis coordinately or postpone cytokinesis for several rounds of chromosome replication, and are well recognized. Despite this surprising biology, the molecular machinery required to achieve this flexibility is largely unknown. In this study, we provide comprehensive experimental evidence that apicomplexan parasites utilize multiple Cdk-related kinases (Crks) to coordinate cell division. We determined that Toxoplasma gondii encodes seven atypical P-, H-, Y- and L- type cyclins and ten Crks to regulate cellular processes. We generated and analyzed conditional tet-OFF mutants for seven TgCrks and four TgCyclins that are expressed in the tachyzoite stage. These experiments demonstrated that TgCrk1, TgCrk2, TgCrk4 and TgCrk6, were required or essential for tachyzoite growth revealing a remarkable number of Crk factors that are necessary for parasite replication. G1 phase arrest resulted from the loss of cytoplasmic TgCrk2 that interacted with a P-type cyclin demonstrating that an atypical mechanism controls half the T. gondii cell cycle. We showed that T. gondii employs at least three TgCrks to complete mitosis. Novel kinases, TgCrk6 and TgCrk4 were required for spindle function and centrosome duplication, respectively, while TgCrk1 and its partner TgCycL were essential for daughter bud assembly. Intriguingly, mitotic kinases TgCrk4 and TgCrk6 did not interact with any cyclin tested and were instead dynamically expressed during mitosis indicating they may not require a cyclin timing mechanism. Altogether, our findings demonstrate that apicomplexan parasites utilize distinctive and complex mechanisms to coordinate their novel replicative cycles.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Gubbels MJ, White M, Szatanek T. The cell cycle and Toxoplasma gondii cell division: tightly knit or loosely stitched? Int J Parasitol. 2008;38(12):1343–58. doi: 10.1016/j.ijpara.2008.06.004 - DOI - PubMed
-
- Francia ME, Striepen B. Cell division in apicomplexan parasites. Nature reviews Microbiology. 2014;12(2):125–36. doi: 10.1038/nrmicro3184 - DOI - PubMed
-
- Suvorova ES, Francia M, Striepen B, White MW. A novel bipartite centrosome coordinates the apicomplexan cell cycle. PLoS Biol. 2015;13(3):e1002093 doi: 10.1371/journal.pbio.1002093 - DOI - PMC - PubMed
-
- Behnke MS, Wootton JC, Lehmann MM, Radke JB, Lucas O, Nawas J, et al. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS One. 2010;5(8):e12354 doi: 10.1371/journal.pone.0012354 - DOI - PMC - PubMed
-
- Blader IJ, Coleman BI, Chen CT, Gubbels MJ. Lytic Cycle of Toxoplasma gondii: 15 Years Later. Annu Rev Microbiol. 2015;69:463–85. doi: 10.1146/annurev-micro-091014-104100 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

