Modern-day SIV viral diversity generated by extensive recombination and cross-species transmission
- PMID: 28672035
- PMCID: PMC5510905
- DOI: 10.1371/journal.ppat.1006466
Modern-day SIV viral diversity generated by extensive recombination and cross-species transmission
Abstract
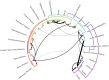
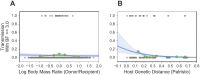
Cross-species transmission (CST) has led to many devastating epidemics, but is still a poorly understood phenomenon. HIV-1 and HIV-2 (human immunodeficiency virus 1 and 2), which have collectively caused over 35 million deaths, are the result of multiple CSTs from chimpanzees, gorillas, and sooty mangabeys. While the immediate history of HIV is known, there are over 45 lentiviruses that infect specific species of primates, and patterns of host switching are not well characterized. We thus took a phylogenetic approach to better understand the natural history of SIV recombination and CST. We modeled host species as a discrete character trait on the viral phylogeny and inferred historical host switches and the pairwise transmission rates between each pair of 24 primate hosts. We identify 14 novel, well-supported, ancient cross-species transmission events. We also find that lentiviral lineages vary widely in their ability to infect new host species: SIVcol (from colobus monkeys) is evolutionarily isolated, while SIVagms (from African green monkeys) frequently move between host subspecies. We also examine the origins of SIVcpz (the predecessor of HIV-1) in greater detail than previous studies, and find that there are still large portions of the genome with unknown origins. Observed patterns of CST are likely driven by a combination of ecological circumstance and innate immune factors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa.Front Biosci. 2004 Jan 1;9:225-54. doi: 10.2741/1154. Front Biosci. 2004. PMID: 14766362 Review.
-
Phyloepidemiological Analysis Reveals that Viral Divergence Led to the Paucity of Simian Immunodeficiency Virus SIVmus/gsn/mon Infections in Wild Populations.J Virol. 2017 Feb 28;91(6):e01884-16. doi: 10.1128/JVI.01884-16. Print 2017 Mar 15. J Virol. 2017. PMID: 28077632 Free PMC article.
-
Discovery and full genome characterization of two highly divergent simian immunodeficiency viruses infecting black-and-white colobus monkeys (Colobus guereza) in Kibale National Park, Uganda.Retrovirology. 2013 Oct 21;10:107. doi: 10.1186/1742-4690-10-107. Retrovirology. 2013. PMID: 24139306 Free PMC article.
-
Evolution of the uniquely adaptable lentiviral envelope in a natural reservoir host.Retrovirology. 2006 Mar 20;3:19. doi: 10.1186/1742-4690-3-19. Retrovirology. 2006. PMID: 16549011 Free PMC article.
-
Phylogeny and natural history of the primate lentiviruses, SIV and HIV.Curr Opin Genet Dev. 1995 Dec;5(6):798-806. doi: 10.1016/0959-437x(95)80014-v. Curr Opin Genet Dev. 1995. PMID: 8745080 Review.
Cited by
-
Examination of the APOBEC3 Barrier to Cross Species Transmission of Primate Lentiviruses.Viruses. 2021 Jun 7;13(6):1084. doi: 10.3390/v13061084. Viruses. 2021. PMID: 34200141 Free PMC article. Review.
-
Noninvasive western lowland gorilla's health monitoring: A decade of simian immunodeficiency virus surveillance in southern Cameroon.Ecol Evol. 2018 Oct 25;8(22):10698-10710. doi: 10.1002/ece3.4478. eCollection 2018 Nov. Ecol Evol. 2018. PMID: 30519399 Free PMC article.
-
Cryo-EM structures of prefusion SIV envelope trimer.Nat Struct Mol Biol. 2022 Nov;29(11):1080-1091. doi: 10.1038/s41594-022-00852-1. Epub 2022 Nov 7. Nat Struct Mol Biol. 2022. PMID: 36344847 Free PMC article.
-
Species-specific vulnerability of RanBP2 shaped the evolution of SIV as it transmitted in African apes.PLoS Pathog. 2018 Mar 8;14(3):e1006906. doi: 10.1371/journal.ppat.1006906. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29518153 Free PMC article.
-
Addressing the illegal wildlife trade in the European Union as a public health issue to draw decision makers attention.Biol Conserv. 2020 Nov;251:108798. doi: 10.1016/j.biocon.2020.108798. Epub 2020 Oct 12. Biol Conserv. 2020. PMID: 33071292 Free PMC article. Review.
References
-
- Morens D, Folkers G, Fauci A. Emerging infections: a perpetual challenge In: LANCET INFECTIOUS DISEASES [Internet]. 2008. [cited 11 Aug 2015] pp. 710–719. doi: 10.1016/S1473-3099(08)70256-1 - DOI - PMC - PubMed
-
- Parrish CR, Holmes EC, Morens DM, Park E-C, Burke DS, Calisher CH, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev. 2008;72: 457–70. doi: 10.1128/MMBR.00004-08 - DOI - PMC - PubMed
-
- Locatelli S, Peeters M. Cross-species transmission of simian retroviruses: how and why they could lead to the emergence of new diseases in the human population. AIDS. 2012;26: 659–73. doi: 10.1097/QAD.0b013e328350fb68 - DOI - PubMed
-
- Apetrei C, Robertson DL, Marx PA. The history of SIVS and AIDS: epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front Biosci. 2004;9: 225–54. Available: http://www.ncbi.nlm.nih.gov/pubmed/14766362 - PubMed
-
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1: a006841 doi: 10.1101/cshperspect.a006841 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

