Sphingosine-1-phosphate suppresses chondrosarcoma metastasis by upregulation of tissue inhibitor of metalloproteinase 3 through suppressing miR-101 expression
- PMID: 28672103
- PMCID: PMC5623823
- DOI: 10.1002/1878-0261.12106
Sphingosine-1-phosphate suppresses chondrosarcoma metastasis by upregulation of tissue inhibitor of metalloproteinase 3 through suppressing miR-101 expression
Abstract
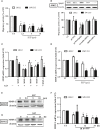
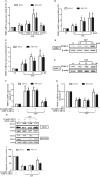
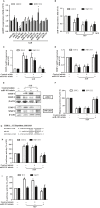
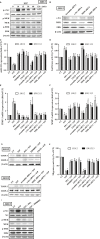
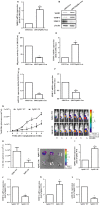
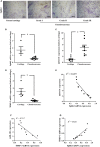
Chondrosarcoma is the second most common primary malignancy form of bone cancer, exhibiting resistance to chemotherapy and radiation therapy as well as developing high metastasis ability in late-stage tumors. Thus, understanding the metastatic processes of chondrosarcoma is considered a strategy for the treatment of this disease. Sphingosine 1-phosphate (S1P), a bioactive sphingolipid, is produced intracellularly by sphingosine kinase (SphK) and is regarded as a second signaling molecule that regulates inflammation, proliferation, angiogenesis, and metastasis. However, the effect of S1P on chondrosarcoma remains uncertain. As demonstrated by the transwell, immunoblotting, and real-time PCR analyses, we found that S1P inhibited cell migration and MMP-2 expression through the upregulation of the tissue inhibitor of metalloproteinase-3 (TIMP-3) expression in human chondrosarcoma cells. Additionally, we also showed that microRNA (miRNA)-101, which targets the 3' untranslated region (3'UTR) of TIMP-3, decreased significantly following S1P treatment. After transfection with miR-101 mimics, the S1P-regulated cell migration and TIMP-3 expression were both reversed. Furthermore, we also showed that the S1P-inhibited cell migration is mediated through the c-Src/MEK/ERK signaling axis. Meanwhile, the in vivo study indicated that overexpression of SphK1 decreases chondrosarcoma metastasis to the lungs. Our results illustrate the clinical significance between SphK1, TIMP-3, and miR-101 in human chondrosarcoma patients. Taken together, our results suggest that S1P and miR-101 may prove to be potential therapeutic targets for future chondrosarcoma treatment.
Keywords: chondrosarcoma; metastasis; microRNA; sphingosine-1-phosphate; tissue inhibitor of metalloproteinase.
© 2017 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Figures
Similar articles
-
Sphingosine-1-phosphate promotes PDGF-dependent endothelial progenitor cell angiogenesis in human chondrosarcoma cells.Aging (Albany NY). 2019 Dec 6;11(23):11040-11053. doi: 10.18632/aging.102508. Epub 2019 Dec 6. Aging (Albany NY). 2019. PMID: 31809267 Free PMC article.
-
SphK1/S1P Mediates PDGF-Induced Pulmonary Arterial Smooth Muscle Cell Proliferation via miR-21/BMPRII/Id1 Signaling Pathway.Cell Physiol Biochem. 2018;51(1):487-500. doi: 10.1159/000495243. Epub 2018 Nov 19. Cell Physiol Biochem. 2018. PMID: 30453304
-
Transforming growth factor-β-sphingosine kinase 1/S1P signaling upregulates microRNA-21 to promote fibrosis in renal tubular epithelial cells.Exp Biol Med (Maywood). 2016 Feb;241(3):265-72. doi: 10.1177/1535370215605586. Epub 2015 Sep 16. Exp Biol Med (Maywood). 2016. PMID: 26376826 Free PMC article.
-
Targeting sphingosine-1-phosphate signaling for cancer therapy.Sci China Life Sci. 2017 Jun;60(6):585-600. doi: 10.1007/s11427-017-9046-6. Epub 2017 May 27. Sci China Life Sci. 2017. PMID: 28623546 Review.
-
Sphingosine Kinase 1 and Sphingosine-1-Phosphate Signaling in Colorectal Cancer.Int J Mol Sci. 2017 Oct 8;18(10):2109. doi: 10.3390/ijms18102109. Int J Mol Sci. 2017. PMID: 28991193 Free PMC article. Review.
Cited by
-
Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells.Cell Death Dis. 2019 Jan 10;10(1):31. doi: 10.1038/s41419-018-1241-2. Cell Death Dis. 2019. PMID: 30631040 Free PMC article.
-
Unraveling the Mechanism of Xiaochaihu Granules in Alleviating Yeast-Induced Fever Based on Network Analysis and Experimental Validation.Pharmaceuticals (Basel). 2024 Apr 8;17(4):475. doi: 10.3390/ph17040475. Pharmaceuticals (Basel). 2024. PMID: 38675434 Free PMC article.
-
Visfatin-Induced Inhibition of miR-1264 Facilitates PDGF-C Synthesis in Chondrosarcoma Cells and Enhances Endothelial Progenitor Cell Angiogenesis.Cells. 2022 Nov 2;11(21):3470. doi: 10.3390/cells11213470. Cells. 2022. PMID: 36359873 Free PMC article.
-
CXCL13/CXCR5 Interaction Facilitates VCAM-1-Dependent Migration in Human Osteosarcoma.Int J Mol Sci. 2020 Aug 24;21(17):6095. doi: 10.3390/ijms21176095. Int J Mol Sci. 2020. PMID: 32847038 Free PMC article.
-
CCN6-mediated MMP-9 activation enhances metastatic potential of human chondrosarcoma.Cell Death Dis. 2018 Sep 20;9(10):955. doi: 10.1038/s41419-018-1008-9. Cell Death Dis. 2018. PMID: 30237403 Free PMC article.
References
-
- Arpino V, Brock M and Gill SE (2015) The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol 44–46, 247–s254. - PubMed
-
- Baker AH, Edwards DR and Murphy G (2002) Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 115, 3719–3727. - PubMed
-
- Chang CL, Ho MC, Lee PH, Hsu CY, Huang WP and Lee H (2009) S1P(5) is required for sphingosine 1‐phosphate‐induced autophagy in human prostate cancer PC‐3 cells. Am J Physiol Cell Physiol 297, C451–C458. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

