Sugars, Sweet Taste Receptors, and Brain Responses
- PMID: 28672790
- PMCID: PMC5537773
- DOI: 10.3390/nu9070653
Sugars, Sweet Taste Receptors, and Brain Responses
Abstract
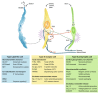
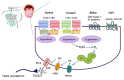
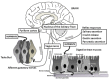
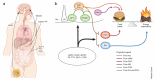
Sweet taste receptors are composed of a heterodimer of taste 1 receptor member 2 (T1R2) and taste 1 receptor member 3 (T1R3). Accumulating evidence shows that sweet taste receptors are ubiquitous throughout the body, including in the gastrointestinal tract as well as the hypothalamus. These sweet taste receptors are heavily involved in nutrient sensing, monitoring changes in energy stores, and triggering metabolic and behavioral responses to maintain energy balance. Not surprisingly, these pathways are heavily regulated by external and internal factors. Dysfunction in one or more of these pathways may be important in the pathogenesis of common diseases, such as obesity and type 2 diabetes mellitus.
Keywords: glucose sensing; hypothalamus; leptin; nutrient sensing; sweet taste receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Janssen S., Depoortere I. Nutrient sensing in the gut: New roads to therapeutics? Trends Endocrinol. Metab. 2013;24:92–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

