Immune Modulating Topical S100A8/A9 Inhibits Growth of Pseudomonas aeruginosa and Mitigates Biofilm Infection in Chronic Wounds
- PMID: 28672877
- PMCID: PMC5535852
- DOI: 10.3390/ijms18071359
Immune Modulating Topical S100A8/A9 Inhibits Growth of Pseudomonas aeruginosa and Mitigates Biofilm Infection in Chronic Wounds
Abstract
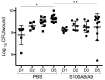
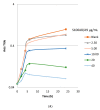
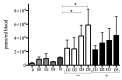
Pseudomonas aeruginosa biofilm maintains and perturbs local host defense, hindering timely wound healing. Previously, we showed that P. aeruginosa suppressed S100A8/A9 of the murine innate host defense. We assessed the potential antimicrobial effect of S100A8/A9 on biofilm-infected wounds in a murine model and P. aeruginosa growth in vitro. Seventy-six mice, inflicted with a full-thickness burn wound were challenged subcutaneously (s.c.) by 10⁶ colony-forming units (CFUs) of P. aeruginosa biofilm. Mice were subsequently randomized into two treatment groups, one group receiving recombinant murine S100A8/A9 and a group of vehicle controls (phosphate-buffered saline, PBS) all treated with s.c. injections daily for up to five days. Wounds were analyzed for quantitative bacteriology and contents of key inflammatory markers. Count of blood polymorphonuclear leukocytes was included. S100A8/A9-treatment ameliorated wound infection, as evaluated by quantitative bacteriology (p ≤ 0.05). In vitro, growth of P. aeruginosa was inhibited dose-dependently by S100A8/A9 in concentrations from 5 to 40 μg/mL, as determined by optical density-measurement (OD-measurement) and quantitative bacteriology. Treatment slightly augmented key inflammatory cytokine Tumor Necrosis Factor-α (TNF-α), but dampened interferon-γ (IFN-γ) levels and blood polymorphonuclear count. In conclusion, topical S100A8/A9 displays remarkable novel immune stimulatory and anti-infective properties in vivo and in vitro. Importantly, treatment by S100A8/A9 provides local infection control. Implications for a role as adjunctive treatment in healing of chronic biofilm-infected wounds are discussed.
Keywords: Pseudomonas aeruginosa; S100A8/A9; biofilm infection; chronic wounds; host defense.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Malone M., Bjarnsholt T., McBain A.J., James G.A., Stoodley P., Leaper D., Tachi M., Schultz G., Swanson T., Wolcott R.D. The prevalence of biofilms in chronic wounds: A systematic review and meta-analysis of published data. J. Wound Care. 2017;26:20–25. doi: 10.12968/jowc.2017.26.1.20. - DOI - PubMed
-
- Trøstrup H., Bjarnsholt T., Kirketerp-Møller K., Høiby N., Moser C. What is new in the understanding of non healing wounds. Ulcers. 2013;2013:625934. doi: 10.1155/2013/625934. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

