A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications
- PMID: 28673039
- PMCID: PMC6287254
- DOI: 10.1210/er.2017-00025
A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications
Abstract
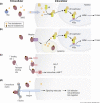
In the circulation, testosterone and other sex hormones are bound to binding proteins, which play an important role in regulating their transport, distribution, metabolism, and biological activity. According to the free hormone hypothesis, which has been debated extensively, only the unbound or free fraction is biologically active in target tissues. Consequently, accurate determination of the partitioning of testosterone between bound and free fractions is central to our understanding of how its delivery to the target tissues and biological activity are regulated and consequently to the diagnosis and treatment of androgen disorders in men and women. Here, we present a historical perspective on the evolution of our understanding of the binding of testosterone to circulating binding proteins. On the basis of an appraisal of the literature as well as experimental data, we show that the assumptions of stoichiometry, binding dynamics, and the affinity of the prevailing models of testosterone binding to sex hormone-binding globulin and human serum albumin are not supported by published experimental data and are most likely inaccurate. This review offers some guiding principles for the application of free testosterone measurements in the diagnosis and treatment of patients with androgen disorders. The growing number of testosterone prescriptions and widely recognized problems with the direct measurement as well as the computation of free testosterone concentrations render this critical review timely and clinically relevant.
Copyright © 2017 Endocrine Society.
Figures

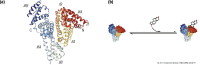

Comment in
-
Free Testosterone: Pumping up the Tires or Ending the Free Ride?Endocr Rev. 2017 Aug 1;38(4):297-301. doi: 10.1210/er.2017-00171. Endocr Rev. 2017. PMID: 28898980 No abstract available.
References
-
- Manni A, Pardridge WM, Cefalu W, Nisula BC, Bardin CW, Santner SJ, Santen RJ. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab. 1985;61(4):705–710. - PubMed
-
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. - PubMed
-
- Södergard R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16(6):801–810. - PubMed
-
- Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

