Effect of a 6-month pedometer-based walking intervention on functional capacity in patients with chronic heart failure with reduced (HFrEF) and with preserved (HFpEF) ejection fraction: study protocol for two multicenter randomized controlled trials
- PMID: 28673328
- PMCID: PMC5496141
- DOI: 10.1186/s12967-017-1257-x
Effect of a 6-month pedometer-based walking intervention on functional capacity in patients with chronic heart failure with reduced (HFrEF) and with preserved (HFpEF) ejection fraction: study protocol for two multicenter randomized controlled trials
Abstract
Background: Regular physical activity is recommended for patients with chronic heart failure to improve their functional capacity, and walking is a popular, effective, and safe form of physical activity. Pedometers have shown potential to increase the amount of walking across a range of chronic diseases, but it is unknown whether a pedometer-based intervention improves functional capacity and neurohumoral modulation in heart failure patients.
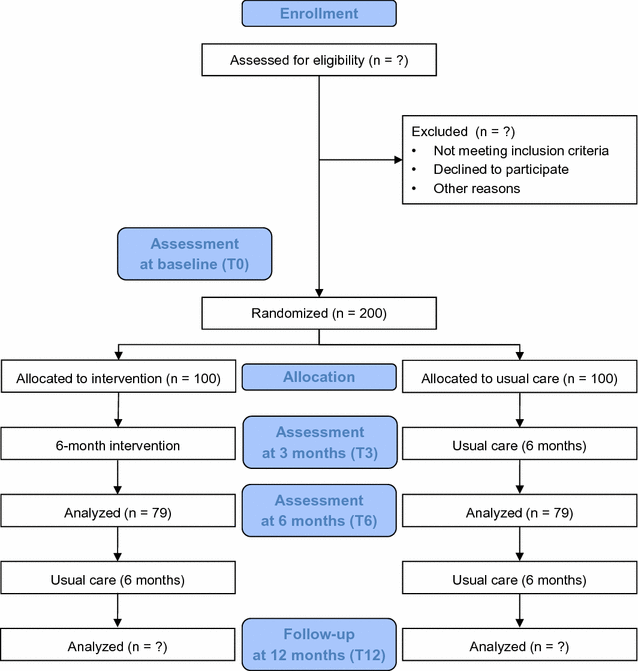
Methods: Two multicenter randomized controlled trials will be conducted in parallel: one in patients with chronic heart failure with reduced ejection fraction (HFrEF), the other in patients with chronic heart failure with preserved ejection fraction (HFpEF). Each trial will consist of a 6-month intervention with an assessment at baseline, at 3 months, at the end of the intervention, and 6 months after completing the intervention. Each trial will aim to include a total of 200 physically inactive participants with chronic heart failure who will be randomly assigned to intervention or control arms. The 6-month intervention will consist of an individualized pedometer-based walking program with weekly step goals, behavioral face-to-face sessions with a physician, and regular telephone calls with a research nurse. The intervention will be based on effective behavioral principles (goal setting, self-monitoring, personalized feedback). The primary outcome is the change in 6-min walk distance at the end of the 6-month intervention. Secondary outcomes include changes in serum biomarkers levels, pulmonary congestion assessed by ultrasound, average daily step count measured by accelerometry, anthropometric measures, symptoms of depression, health-related quality of life, self-efficacy, and MAGGIC risk score.
Discussion: To our knowledge, these are the first studies to evaluate a pedometer-based walking intervention in patients with chronic heart failure with either reduced or preserved ejection fraction. The studies will contribute to a better understanding of physical activity promotion in heart failure patients to inform future physical activity recommendations and heart failure guidelines. Trial registration The trials are registered in ClinicalTrials.gov, identifiers: NCT03041610, registered 29 January 2017 (HFrEF), NCT03041376, registered 1 February 2017 (HFpEF).
Keywords: 6-min walk test; Chronic heart failure; Functional capacity; NT-proBNP; Pedometer; Physical activity; Walking.
References
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials