Sustained hyperosmolarity increses TGF-ß1 and Egr-1 expression in the rat renal medulla
- PMID: 28673338
- PMCID: PMC5496335
- DOI: 10.1186/s12882-017-0626-2
Sustained hyperosmolarity increses TGF-ß1 and Egr-1 expression in the rat renal medulla
Abstract
Background: Although TGF-ß and the transcription factor Egr-1 play an important role in both kidney fibrosis and in response to acute changes of renal medullary osmolarity, their role under sustained hypo- or hyperosmolar conditions has not been elucidated. We investigated the effects of chronic hypertonicity and hypotonicity on the renal medullary TGF-ß and Egr-1 expression.
Methods: Male adult Sprague Dawley rats (n = 6/group) were treated with 15 mg/day furosemide, or the rats were water restricted to 15 ml/200 g body weight per day. Control rats had free access to water and rodent chow. Kidneys were harvested after 5 days of treament. In cultured inner medullary collecting duct (IMCD) cells, osmolarity was increased from 330 mOsm to 900 mOsm over 6 days. Analyses were performed at 330, 600 and 900 mOsm.
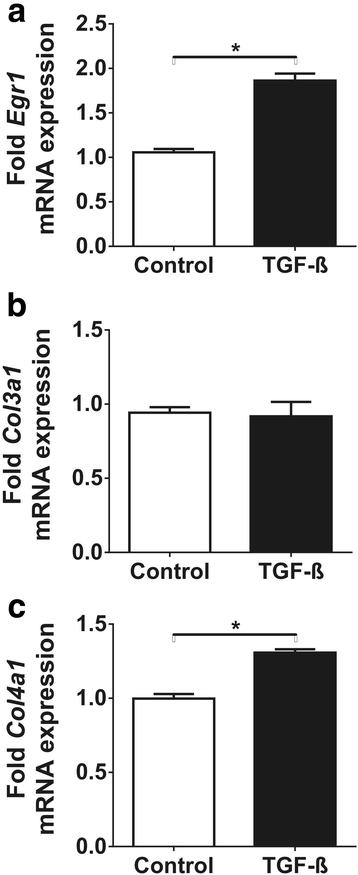
Results: Urine osmolarity has not changed due to furosemide treatment but increased 2-fold after water restriction (p < 0.05). Gene expression of TGF-ß and Egr-1 increased by 1.9-fold and 7-fold in the hypertonic medulla, respectively (p < 0.05), accompanied by 6-fold and 2-fold increased c-Fos and TIMP-1 expression, respectively (p < 0.05) and positive immunostaining for TGF-ß and Egr-1 (p < 0.05). Similarly, hyperosmolarity led to overexpression of TGF-ß and Egr-1 mRNA in IMCD cells (2.5-fold and 3.5-fold increase from 330 to 900 mOsm, respectively (p < 0.05)) accompanied by significant c-Fos and c-Jun overexpressions (p < 0.01), and increased Col3a1 and Col4a1 mRNA expression.
Conclusion: We conclude that both TGF-ß and Egr-1 are upregulated by sustained hyperosmolarity in the rat renal medulla, and it favors the expression of extracellular matrix components.
Keywords: Egr-1; Fibrosis; Osmotic stress; Sodium chloride; TGF-ß; Urea.
Conflict of interest statement
Ethics approval
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and the European Directive 2010/63/EU. All procedures and handling of animals during the investigations were reviewed and approved by the local Ethical Committee for Animal Experimentation at Semmelweis University and the Directorate of Food Chain Safety and Animal Health of the Pest County Government Office (permission number XIV-I-001/2104–4/2012). Treatment of animals was in accordance with the Arrive Guidelines for Reporting Animal Research (Additional file 1: ARRIVE Checklist).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
Hypotonicity increases transcription, expression, and action of Egr-1 in murine renal medullary mIMCD3 cells.Am J Physiol. 1997 Nov;273(5):F837-42. doi: 10.1152/ajprenal.1997.273.5.F837. Am J Physiol. 1997. PMID: 9374849
-
Oxygen-dependent expression of hypoxia-inducible factor-1alpha in renal medullary cells of rats.Physiol Genomics. 2001 Aug 28;6(3):159-68. doi: 10.1152/physiolgenomics.2001.6.3.159. Physiol Genomics. 2001. PMID: 11526200
-
DOCA and TGF-beta induce early growth response gene-1 (Egr-1) expression.Cell Physiol Biochem. 2008;22(5-6):465-74. doi: 10.1159/000185495. Epub 2008 Dec 9. Cell Physiol Biochem. 2008. PMID: 19088428
-
[Molecular mechanisms of nephro-protective action of enalapril in experimental chronic renal failure].Ann Acad Med Stetin. 1999;Suppl 52:1-93. Ann Acad Med Stetin. 1999. PMID: 10589103 Review. Polish.
-
Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy.Matrix Biol. 2011 May;30(4):235-42. doi: 10.1016/j.matbio.2011.03.005. Epub 2011 Apr 13. Matrix Biol. 2011. PMID: 21511034 Free PMC article. Review.
Cited by
-
Iguratimod attenuated fibrosis in systemic sclerosis via targeting early growth response 1 expression.Arthritis Res Ther. 2023 Aug 18;25(1):151. doi: 10.1186/s13075-023-03135-2. Arthritis Res Ther. 2023. PMID: 37596660 Free PMC article.
-
UT-A1/A3 knockout mice show reduced fibrosis following unilateral ureteral obstruction.Am J Physiol Renal Physiol. 2020 May 1;318(5):F1160-F1166. doi: 10.1152/ajprenal.00008.2020. Epub 2020 Mar 16. Am J Physiol Renal Physiol. 2020. PMID: 32174141 Free PMC article.
-
The PPARγ agonist pioglitazone prevents TGF-β induced renal fibrosis by repressing EGR-1 and STAT3.BMC Nephrol. 2019 Jul 5;20(1):245. doi: 10.1186/s12882-019-1431-x. BMC Nephrol. 2019. PMID: 31277592 Free PMC article.
-
Genetic or siRNA inhibition of MBD2 attenuates the UUO- and I/R-induced renal fibrosis via downregulation of EGR1.Mol Ther Nucleic Acids. 2022 Feb 28;28:77-86. doi: 10.1016/j.omtn.2022.02.015. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35356685 Free PMC article.
-
Pioglitazone Protects Tubular Epithelial Cells during Kidney Fibrosis by Attenuating miRNA Dysregulation and Autophagy Dysfunction Induced by TGF-β.Int J Mol Sci. 2023 Oct 24;24(21):15520. doi: 10.3390/ijms242115520. Int J Mol Sci. 2023. PMID: 37958504 Free PMC article.
References
-
- Floege J, Alpers CE, Burns MW, Pritzl P, Gordon K, Couser WG, et al. Glomerular cells, extracellular matrix accumulation, and the development of glomerulosclerosis in the remnant kidney model. Laboratory investigation. a journal of technical methods and pathology. 1992;66:485–497. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

