ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: Role of Src-family kinases and ARHGEF1
- PMID: 28673614
- PMCID: PMC5542024
- DOI: 10.1016/j.freeradbiomed.2017.06.022
ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: Role of Src-family kinases and ARHGEF1
Abstract
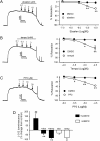
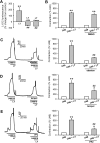
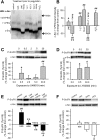
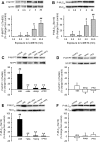
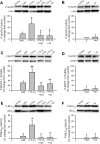
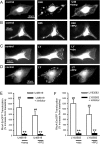
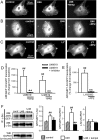
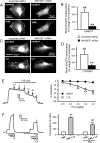
The role of reactive oxygen species (ROS) in smooth muscle contraction is poorly understood. We hypothesised that G-protein coupled receptor (GPCR) activation and hypoxia induce Rho-kinase activity and contraction in rat intra-pulmonary artery (IPA) via stimulation of ROS production and subsequent Src-family kinase (SrcFK) activation. The T-type prostanoid receptor agonist U46619 induced ROS production in pulmonary artery smooth muscle cells (PASMC). U46619 also induced c-Src cysteine oxidation, SrcFK auto-phosphorylation, MYPT-1 and MLC20 phosphorylation and contraction in IPA, and all these responses were inhibited by antioxidants (ebselen, Tempol). Contraction and SrcFK/MYPT-1/MLC20 phosphorylations were also inhibited by combined superoxide dismutase and catalase, or by the SrcFK antagonist PP2, while contraction and MYPT-1/MLC20 phosphorylations were inhibited by the Rho guanine nucleotide exchange factor (RhoGEF) inhibitor Y16. H2O2 and the superoxide-generating quinoledione LY83583 both induced c-Src oxidation, SrcFK auto-phosphorylation and contraction in IPA. LY83583 and H2O2-induced contractions were inhibited by PP2, while LY83583-induced contraction was also inhibited by antioxidants and Y16. SrcFK auto-phosphorylation and MYPT-1/MLC20 phosphorylation was also induced by hypoxia in IPA and this was blocked by mitochondrial inhibitors rotenone and myxothiazol. In live PASMC, sub-cellular translocation of RhoA and the RhoGEF ARHGEF1 was triggered by both U46619 and LY83583 and this translocation was blocked by antioxidants and PP2. RhoA translocation was also inhibited by an ARHGEF1 siRNA. U46619 enhanced ROS-dependent co-immunoprecipitation of ARHGEF1 with c-Src. Our results demonstrate a link between GPCR-induced cytosolic ROS or hypoxia-induced mitochondrial ROS and SrcFK activity, Rho-kinase activity and contraction. ROS and SrcFK activate RhoA via ARHGEF1.
Keywords: Guanine nucleotide exchange factors; Hypoxia; Pulmonary artery; Reactive oxygen species; Rho-kinase; Src-family kinases; Tyrosine kinases; Vascular smooth muscle.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Transforming growth factor-β enhances Rho-kinase activity and contraction in airway smooth muscle via the nucleotide exchange factor ARHGEF1.J Physiol. 2018 Jan 1;596(1):47-66. doi: 10.1113/JP275033. Epub 2017 Nov 23. J Physiol. 2018. PMID: 29071730 Free PMC article.
-
Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery.Cardiovasc Res. 2008 Feb 1;77(3):570-9. doi: 10.1093/cvr/cvm073. Epub 2007 Nov 21. Cardiovasc Res. 2008. PMID: 18032393 Free PMC article.
-
Divergent modulation of Rho-kinase and Ca(2+) influx pathways by Src family kinases and focal adhesion kinase in airway smooth muscle.Br J Pharmacol. 2015 Nov;172(22):5265-80. doi: 10.1111/bph.13313. Epub 2015 Oct 23. Br J Pharmacol. 2015. PMID: 26294392 Free PMC article.
-
Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery.Cardiovasc Res. 2008 Dec 1;80(3):453-62. doi: 10.1093/cvr/cvn209. Epub 2008 Aug 5. Cardiovasc Res. 2008. PMID: 18682436 Free PMC article.
-
Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease.J Physiol. 2015 Sep 1;593(17):3815-28. doi: 10.1113/jphysiol.2014.285304. Epub 2014 Dec 1. J Physiol. 2015. PMID: 25384773 Free PMC article. Review.
Cited by
-
Targeting the gut microbiota with dietary fibers: a novel approach to prevent the development cardiovascular complications linked to systemic lupus erythematosus in a preclinical study.Gut Microbes. 2023 Dec;15(2):2247053. doi: 10.1080/19490976.2023.2247053. Gut Microbes. 2023. PMID: 37615336 Free PMC article.
-
The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure.Nutrients. 2019 Apr 16;11(4):857. doi: 10.3390/nu11040857. Nutrients. 2019. PMID: 30995745 Free PMC article.
-
P2X7 receptor activation aggravates NADPH oxidase 2-induced oxidative stress after intracerebral hemorrhage.Neural Regen Res. 2021 Aug;16(8):1582-1591. doi: 10.4103/1673-5374.303036. Neural Regen Res. 2021. PMID: 33433488 Free PMC article.
-
Corneal Edema in Inducible Slc4a11 Knockout Is Initiated by Mitochondrial Superoxide Induced Src Kinase Activation.Cells. 2023 Jun 1;12(11):1528. doi: 10.3390/cells12111528. Cells. 2023. PMID: 37296649 Free PMC article.
-
Gut Microbiota Has a Crucial Role in the Development of Hypertension and Vascular Dysfunction in Toll-like Receptor 7-Driven Lupus Autoimmunity.Antioxidants (Basel). 2021 Sep 7;10(9):1426. doi: 10.3390/antiox10091426. Antioxidants (Basel). 2021. PMID: 34573058 Free PMC article.
References
-
- Fomenko D.E., Xing W., Adair B.M., Thomas D.J., Gladyshev V.N. High-throughput identification of catalytic redox-active cysteine residues. Science. 2007;315:387–389. - PubMed
-
- Knock G.A., Ward J.P. Redox regulation of protein kinases as a modulator of vascular function. Antioxid. Redox Signal. 2011;15:1531–1547. - PubMed
-
- Miki H., Funato Y. Regulation of intracellular signalling through cysteine oxidation by reactive oxygen species. J. Biochem. 2012;151:255–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

