The effects of chemical fixation on the cellular nanostructure
- PMID: 28673821
- PMCID: PMC5726765
- DOI: 10.1016/j.yexcr.2017.06.022
The effects of chemical fixation on the cellular nanostructure
Abstract
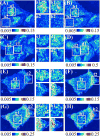
Chemical fixation is nearly indispensable in the biological sciences, especially in circumstances where cryo-fixation is not applicable. While universally employed for the preservation of cell organization, chemical fixatives often introduce artifacts that can confound identification of true structures. Since biological research is increasingly probing ever-finer details of the cellular architecture, it is critical to understand the nanoscale transformation of the cellular organization due to fixation both systematically and quantitatively. In this work, we employed Partial Wave Spectroscopic (PWS) Microscopy, a nanoscale sensitive and label-free live cell spectroscopic-imaging technique, to analyze the effects of the fixation process through three commonly used fixation protocols for cells in vitro. In each method investigated, we detected dramatic difference in both nuclear and cytoplasmic nanoarchitecture between live and fixed states. But significantly, despite the alterations in cellular nanoscale organizations after chemical fixation, the population differences in chromatin structure (e.g. induced by a specific chemotherapeutic agent) remains. In conclusion, we demonstrated that the nanoscale cellular arrangement observed in fixed cells was fundamentally divorced from that in live cells, thus the quantitative analysis is only meaningful on the population level. This finding highlights the importance of live cell imaging techniques with nanoscale sensitivity or cryo-fixation in the interrogation of cellular structure, to complement more traditional chemical fixation methods.
Keywords: Chromatin nanostructure; Fixation; Partial wave spectroscopy.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Preservation of cellular nano-architecture by the process of chemical fixation for nanopathology.PLoS One. 2019 Jul 22;14(7):e0219006. doi: 10.1371/journal.pone.0219006. eCollection 2019. PLoS One. 2019. PMID: 31329606 Free PMC article.
-
Label-free imaging of the native, living cellular nanoarchitecture using partial-wave spectroscopic microscopy.Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):E6372-E6381. doi: 10.1073/pnas.1608198113. Epub 2016 Oct 4. Proc Natl Acad Sci U S A. 2016. PMID: 27702891 Free PMC article.
-
Feasibility of high pressure freezing with freeze substitution after long-term storage in chemical fixatives.Microsc Res Tech. 2013 Sep;76(9):942-6. doi: 10.1002/jemt.22252. Epub 2013 Jul 1. Microsc Res Tech. 2013. PMID: 23818457
-
Hypothesis for the influence of fixatives on the chromatin patterns of interphase nuclei, based on shrinkage and retraction of nuclear and perinuclear structures.Br J Biomed Sci. 2002;59(2):105-13. Br J Biomed Sci. 2002. PMID: 12113398 Review.
-
Correlative microscopy.Arch Biochem Biophys. 2015 Sep 1;581:98-110. doi: 10.1016/j.abb.2015.05.017. Epub 2015 Jun 10. Arch Biochem Biophys. 2015. PMID: 26072116 Review.
Cited by
-
Preservation of cellular nano-architecture by the process of chemical fixation for nanopathology.PLoS One. 2019 Jul 22;14(7):e0219006. doi: 10.1371/journal.pone.0219006. eCollection 2019. PLoS One. 2019. PMID: 31329606 Free PMC article.
-
Assessment of the Effect of Secondary Fixation on the Structure of Meat Products Prepared for Scanning Electron Microscopy.Foods. 2020 Apr 13;9(4):487. doi: 10.3390/foods9040487. Foods. 2020. PMID: 32295008 Free PMC article.
-
Robust and bias-free localization of individual fixed dipole emitters achieving the Cramér Rao bound for applications in cryo-single molecule localization microscopy.PLoS One. 2022 Feb 4;17(2):e0263500. doi: 10.1371/journal.pone.0263500. eCollection 2022. PLoS One. 2022. PMID: 35120171 Free PMC article.
-
Os(II)-Bridged Polyarginine Conjugates: The Additive Effects of Peptides in Promoting or Preventing Permeation in Cells and Multicellular Tumor Spheroids.Inorg Chem. 2021 Jun 7;60(11):8123-8134. doi: 10.1021/acs.inorgchem.1c00769. Epub 2021 May 12. Inorg Chem. 2021. PMID: 33978399 Free PMC article.
-
Cryo-nanoscale chromosome imaging-future prospects.Biophys Rev. 2020 Oct;12(5):1257-1263. doi: 10.1007/s12551-020-00757-7. Epub 2020 Oct 2. Biophys Rev. 2020. PMID: 33006727 Free PMC article. Review.
References
-
- Santana BP, Nedel F, Perelló Ferrúa C, da Silva AF, Demarco FF, Lenin Villarreal Carreño N. Comparing different methods to fix and to dehydrate cells on alginate hydrogel scaffolds using scanning electron microscopy. Microsc Res Tech. 2015 - PubMed
-
- Studer D, Graber W, Al‐Amoudi A, Eggli P. A new approach for cryofixation by high‐pressure freezing. J Microsc. 2001;203(3):285–294. - PubMed
-
- Müller M, Moor H. Cryofixation of Thick Specimens by High Pressure Freezing, The Science of Biological Specimen Preparation. SEM AFM OÕHare, Chicago, IL. 1984, pp:131–138.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

