Hematopoietic Stem Cell Transplantation for Patients with Mucopolysaccharidosis II
- PMID: 28673849
- PMCID: PMC5659208
- DOI: 10.1016/j.bbmt.2017.06.020
Hematopoietic Stem Cell Transplantation for Patients with Mucopolysaccharidosis II
Abstract
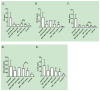
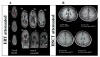
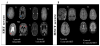
There is limited information regarding the long-term outcomes of hematopoietic stem cell transplantation (HSCT) for mucopolysaccharidosis II (MPS II). In this study, clinical, biochemical, and radiologic findings were assessed in patients who underwent HSCT and/or enzyme replacement therapy (ERT). Demographic data for 146 HSCT patients were collected from 27 new cases and 119 published cases and were compared with 51 ERT and 15 untreated cases. Glycosaminoglycan (GAG) levels were analyzed by liquid chromatography tandem mass spectrometry in blood samples from HSCT, ERT, and untreated patients as well as age-matched controls. Long-term magnetic resonance imaging (MRI) findings were investigated in 13 treated patients (6 ERT and 7 HSCT). Mean age at HSCT was 5.5 years (range, 2 to 21.4 years) in new patients and 5.5 years (range, 10 months to 19.8 years) in published cases. None of the 27 new patients died as a direct result of the HSCT procedure. Graft-versus-host disease occurred in 8 (9%) out of 85 published cases, and 9 (8%) patients died from transplantation-associated complications. Most HSCT patients showed greater improvement in somatic features, joint movements, and activity of daily living than the ERT patients. GAG levels in blood were significantly reduced by ERT and levels were even lower after HSCT. HSCT patients showed either improvement or no progression of abnormal findings in brain MRI while abnormal findings became more extensive after ERT. HSCT seems to be more effective than ERT for MPS II in a wide range of disease manifestations and could be considered as a treatment option for this condition.
Keywords: Enzyme replacement therapy; Hematopoietic stem cell transplantation; Hunter syndrome; Iduronate-2-sulfatase; Mucopolysaccharidosis II.
Copyright © 2017 The American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
References
-
- Neufeld E, Muenzer J. The Mucopolysaccharidoses. New York: McGraw-Hill; 2001.
-
- Tanaka A, Okuyama T, Suzuki Y, et al. Long-term efficacy of hematopoietic stem cell transplantation on brain involvement in patients with mucopolysaccharidosis type II: a nationwide survey in Japan. Mol Genet Metab. 2012;107:513–520. - PubMed
-
- Araya K, Sakai N, Mohri I, et al. Localized donor cells in brain of a Hunter disease patient after cord blood stem cell transplantation. Mol Genet Metab. 2009;98:255–263. - PubMed
-
- Nelson J, Crowhurst J, Carey B, Greed L. Incidence of the mucopolysaccharidoses in Western Australia. Am J Med Genet A. 2003;123A:310–313. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

