Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress
- PMID: 28673968
- PMCID: PMC5530682
- DOI: 10.1073/pnas.1704012114
Hepatic ZIP14-mediated zinc transport is required for adaptation to endoplasmic reticulum stress
Abstract
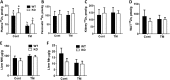
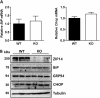
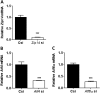
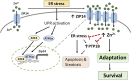
Extensive endoplasmic reticulum (ER) stress damages the liver, causing apoptosis and steatosis despite the activation of the unfolded protein response (UPR). Restriction of zinc from cells can induce ER stress, indicating that zinc is essential to maintain normal ER function. However, a role for zinc during hepatic ER stress is largely unknown despite important roles in metabolic disorders, including obesity and nonalcoholic liver disease. We have explored a role for the metal transporter ZIP14 during pharmacologically and high-fat diet-induced ER stress using Zip14-/- (KO) mice, which exhibit impaired hepatic zinc uptake. Here, we report that ZIP14-mediated hepatic zinc uptake is critical for adaptation to ER stress, preventing sustained apoptosis and steatosis. Impaired hepatic zinc uptake in Zip14 KO mice during ER stress coincides with greater expression of proapoptotic proteins. ER stress-induced Zip14 KO mice show greater levels of hepatic steatosis due to higher expression of genes involved in de novo fatty acid synthesis, which are suppressed in ER stress-induced WT mice. During ER stress, the UPR-activated transcription factors ATF4 and ATF6α transcriptionally up-regulate Zip14 expression. We propose ZIP14 mediates zinc transport into hepatocytes to inhibit protein-tyrosine phosphatase 1B (PTP1B) activity, which acts to suppress apoptosis and steatosis associated with hepatic ER stress. Zip14 KO mice showed greater hepatic PTP1B activity during ER stress. These results show the importance of zinc trafficking and functional ZIP14 transporter activity for adaptation to ER stress associated with chronic metabolic disorders.
Keywords: apoptosis; p-eIF2α/ATF4/CHOP pathway; steatosis; unfolded protein response; zinc metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Activation of G0/G1 switch gene 2 by endoplasmic reticulum stress enhances hepatic steatosis.Metabolism. 2019 Oct;99:32-44. doi: 10.1016/j.metabol.2019.06.015. Epub 2019 Jul 2. Metabolism. 2019. PMID: 31271806
-
Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically induced endoplasmic reticulum stress.Biochem J. 2011 Sep 1;438(2):369-78. doi: 10.1042/BJ20110373. Biochem J. 2011. PMID: 21605081 Free PMC article.
-
Dietary Zinc Regulates Apoptosis through the Phosphorylated Eukaryotic Initiation Factor 2α/Activating Transcription Factor-4/C/EBP-Homologous Protein Pathway during Pharmacologically Induced Endoplasmic Reticulum Stress in Livers of Mice.J Nutr. 2016 Nov;146(11):2180-2186. doi: 10.3945/jn.116.237495. Epub 2016 Sep 7. J Nutr. 2016. PMID: 27605406 Free PMC article.
-
Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function.Am J Physiol Gastrointest Liver Physiol. 2015 Feb 1;308(3):G171-8. doi: 10.1152/ajpgi.00021.2014. Epub 2014 Nov 26. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25428902 Free PMC article. Review.
-
Endoplasmic reticulum stress in liver disease.J Hepatol. 2011 Apr;54(4):795-809. doi: 10.1016/j.jhep.2010.11.005. Epub 2010 Nov 13. J Hepatol. 2011. PMID: 21145844 Free PMC article. Review.
Cited by
-
Different Actions of Intracellular Zinc Transporters ZIP7 and ZIP13 Are Essential for Dermal Development.Int J Mol Sci. 2019 Aug 13;20(16):3941. doi: 10.3390/ijms20163941. Int J Mol Sci. 2019. PMID: 31412620 Free PMC article.
-
Supplementation of Micro- and Macronutrients-A Role of Nutritional Status in Non-Alcoholic Fatty Liver Disease.Int J Mol Sci. 2024 Apr 30;25(9):4916. doi: 10.3390/ijms25094916. Int J Mol Sci. 2024. PMID: 38732128 Free PMC article. Review.
-
Zinc as a countermeasure for cadmium toxicity.Acta Pharmacol Sin. 2021 Mar;42(3):340-346. doi: 10.1038/s41401-020-0396-4. Epub 2020 Apr 13. Acta Pharmacol Sin. 2021. PMID: 32284539 Free PMC article. Review.
-
The role of zinc in the prevention and treatment of nonalcoholic fatty liver disease.Metabol Open. 2021 Jun 29;11:100105. doi: 10.1016/j.metop.2021.100105. eCollection 2021 Sep. Metabol Open. 2021. PMID: 34337376 Free PMC article.
-
MicroRNA-210 Regulates Endoplasmic Reticulum Stress and Apoptosis in Porcine Embryos.Animals (Basel). 2021 Jan 18;11(1):221. doi: 10.3390/ani11010221. Animals (Basel). 2021. PMID: 33477489 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

