Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation
- PMID: 28673989
- PMCID: PMC5530680
- DOI: 10.1073/pnas.1703998114
Quadrupling efficiency in production of genetically modified pigs through improved oocyte maturation
Abstract
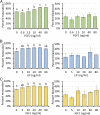
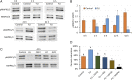
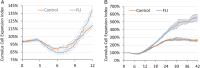
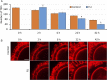
Assisted reproductive technologies in all mammals are critically dependent on the quality of the oocytes used to produce embryos. For reasons not fully clear, oocytes matured in vitro tend to be much less competent to become fertilized, advance to the blastocyst stage, and give rise to live young than their in vivo-produced counterparts, particularly if they are derived from immature females. Here we show that a chemically defined maturation medium supplemented with three cytokines (FGF2, LIF, and IGF1) in combination, so-called "FLI medium," improves nuclear maturation of oocytes in cumulus-oocyte complexes derived from immature pig ovaries and provides a twofold increase in the efficiency of blastocyst production after in vitro fertilization. Transfer of such blastocysts to recipient females doubles mean litter size to about nine piglets per litter. Maturation of oocytes in FLI medium, therefore, effectively provides a fourfold increase in piglets born per oocyte collected. As they progress in culture, the FLI-matured cumulus-oocyte complexes display distinctly different kinetics of MAPK activation in the cumulus cells, much increased cumulus cell expansion, and an accelerated severance of cytoplasmic projections between the cumulus cells outside the zona pellucida and the oocyte within. These events likely underpin the improvement in oocyte quality achieved by using the FLI medium.
Keywords: MAPK signaling; cumulus cell; embryo development; genetic modification; in vitro fertilization.
Conflict of interest statement
Conflict of interest statement: Y.Y., L.D.S., R.S.P., and R.M.R. have a pending patent application “Medium Supplement to Increase the Efficiency of Oocyte maturation and Embryo Culture in vitro.”
Figures

Similar articles
-
Neither gonadotropin nor cumulus cell expansion is needed for the maturation of competent porcine oocytes in vitro†.Biol Reprod. 2021 Aug 3;105(2):533-542. doi: 10.1093/biolre/ioab090. Biol Reprod. 2021. PMID: 33962465
-
FGF2, LIF, and IGF1 (FLI) supplementation during human in vitro maturation enhances markers of gamete competence.Hum Reprod. 2023 Oct 3;38(10):1938-1951. doi: 10.1093/humrep/dead162. Hum Reprod. 2023. PMID: 37608600
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
-
Despite the donor's age, human adipose-derived stem cells enhance the maturation and development rates of porcine oocytes in a co-culture system.Theriogenology. 2018 Jul 15;115:57-64. doi: 10.1016/j.theriogenology.2017.12.024. Epub 2017 Dec 12. Theriogenology. 2018. PMID: 29709724 Review.
-
Regulation of cumulus expansion and hyaluronan synthesis in porcine oocyte-cumulus complexes during in vitro maturation.Endocr Regul. 2012 Oct;46(4):225-35. doi: 10.4149/endo_2012_04_225. Endocr Regul. 2012. PMID: 23127506 Review.
Cited by
-
Copper deficiency affects the developmental competence of porcine oocytes matured in vitro.Front Cell Dev Biol. 2022 Sep 7;10:993030. doi: 10.3389/fcell.2022.993030. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36158185 Free PMC article.
-
PTPN11 (SHP2) Is Indispensable for Growth Factors and Cytokine Signal Transduction During Bovine Oocyte Maturation and Blastocyst Development.Cells. 2019 Oct 18;8(10):1272. doi: 10.3390/cells8101272. Cells. 2019. PMID: 31635340 Free PMC article.
-
Ablation of conceptus PTGS2 expression does not alter early conceptus development and establishment of pregnancy in the pig†.Biol Reprod. 2020 Feb 14;102(2):475-488. doi: 10.1093/biolre/ioz192. Biol Reprod. 2020. PMID: 31616930 Free PMC article.
-
Domesticated cynomolgus monkey embryonic stem cells allow the generation of neonatal interspecies chimeric pigs.Protein Cell. 2020 Feb;11(2):97-107. doi: 10.1007/s13238-019-00676-8. Epub 2019 Nov 28. Protein Cell. 2020. PMID: 31781970 Free PMC article.
-
In vitro maturation using αMEM with reduced NaCl enhances maturation and developmental competence of pig oocytes after somatic cell nuclear transfer.J Vet Sci. 2022 Mar;23(2):e31. doi: 10.4142/jvs.21279. J Vet Sci. 2022. PMID: 35363440 Free PMC article.
References
-
- Yuan Y, Krisher RL. In vitro maturation (IVM) of porcine oocytes. Methods Mol Biol. 2012;825:183–198. - PubMed
-
- Lonergan P, Fair T. Maturation of oocytes in vitro. Annu Rev Anim Biosci. 2016;4:255–268. - PubMed
-
- Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology. 2014;81:24–37. - PubMed
-
- Seyhan A, et al. Severe early ovarian hyperstimulation syndrome following GnRH agonist trigger with the addition of 1500 IU hCG. Hum Reprod. 2013;28:2522–2528. - PubMed
-
- Humaidan P, et al. Ovarian hyperstimulation syndrome: Review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31:1997–2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

