Lack of evidence for involvement of TonEBP and hyperosmotic stimulus in induction of autophagy in the nucleus pulposus
- PMID: 28674405
- PMCID: PMC5495809
- DOI: 10.1038/s41598-017-04876-2
Lack of evidence for involvement of TonEBP and hyperosmotic stimulus in induction of autophagy in the nucleus pulposus
Abstract
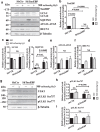
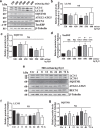
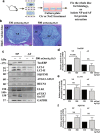
Nucleus pulposus (NP) cells reside in a physiologically hyperosmotic environment within the intervertebral disc. TonEBP/NFAT5 is an osmo-sensitive transcription factor that controls expression of genes critical for cell survival under hyperosmotic conditions. A recent report on NP and studies of other cell types have shown that hyperosmolarity triggers autophagy. However, little is known whether such autophagy induction occurs through TonEBP. The goal of this study was to investigate the role of TonEBP in hyperosmolarity-dependent autophagy in NP. Loss-of-function studies showed that autophagy in NP cells was not TonEBP-dependent; hyperosmolarity did not upregulate autophagy as previously reported. NP tissue of haploinsufficient TonEBP mice showed normal pattern of LC3 staining. NP cells did not increase LC3-II or LC3-positive puncta under hyperosmotic conditions. Bafilomycin-A1 treatment and tandem mCherry-EGFP-LC3B reporter transfection demonstrated that the autophagic flux was unaffected by hyperosmolarity. Even under serum-free conditions, NP cells did not induce autophagy with increasing osmolarity. Hyperosmolarity did not change the phosphorylation of ULK1 by mTOR and AMPK. An ex vivo disc organ culture study supported that extracellular hyperosmolarity plays no role in promoting autophagy in the NP. We conclude that hyperosmolarity does not play a role in autophagy induction in NP cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
RNA Sequencing Reveals a Role of TonEBP Transcription Factor in Regulation of Pro-inflammatory Genes in Response to Hyperosmolarity in Healthy Nucleus Pulposus Cells: A HOMEOSTATIC RESPONSE?J Biol Chem. 2016 Dec 23;291(52):26686-26697. doi: 10.1074/jbc.M116.757732. Epub 2016 Nov 8. J Biol Chem. 2016. PMID: 27875309 Free PMC article.
-
COX-2 expression mediated by calcium-TonEBP signaling axis under hyperosmotic conditions serves osmoprotective function in nucleus pulposus cells.J Biol Chem. 2018 Jun 8;293(23):8969-8981. doi: 10.1074/jbc.RA117.001167. Epub 2018 Apr 26. J Biol Chem. 2018. PMID: 29700115 Free PMC article.
-
Hypoxia promotes noncanonical autophagy in nucleus pulposus cells independent of MTOR and HIF1A signaling.Autophagy. 2016 Sep;12(9):1631-46. doi: 10.1080/15548627.2016.1192753. Epub 2016 Jun 17. Autophagy. 2016. PMID: 27314664 Free PMC article.
-
Extracellular osmolarity regulates matrix homeostasis in the intervertebral disc and articular cartilage: evolving role of TonEBP.Matrix Biol. 2014 Nov;40:10-6. doi: 10.1016/j.matbio.2014.08.014. Epub 2014 Aug 27. Matrix Biol. 2014. PMID: 25172826 Free PMC article. Review.
-
Role of autophagy in intervertebral disc degeneration.J Cell Physiol. 2022 Feb;237(2):1266-1284. doi: 10.1002/jcp.30631. Epub 2021 Nov 17. J Cell Physiol. 2022. PMID: 34787318 Free PMC article. Review.
Cited by
-
HIF1A and NFAT5 coordinate Na+-boosted antibacterial defense via enhanced autophagy and autolysosomal targeting.Autophagy. 2019 Nov;15(11):1899-1916. doi: 10.1080/15548627.2019.1596483. Epub 2019 Apr 14. Autophagy. 2019. PMID: 30982460 Free PMC article.
-
Role of autophagy in intervertebral disc and cartilage function: implications in health and disease.Matrix Biol. 2021 Jun;100-101:207-220. doi: 10.1016/j.matbio.2020.12.002. Epub 2020 Dec 7. Matrix Biol. 2021. PMID: 33301899 Free PMC article. Review.
-
Hyperosmotic Stress Initiates AMPK-Independent Autophagy and AMPK- and Autophagy-Independent Depletion of Thioredoxin 1 and Glyoxalase 2 in HT22 Nerve Cells.Oxid Med Cell Longev. 2019 Mar 27;2019:2715810. doi: 10.1155/2019/2715810. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31049129 Free PMC article.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
Transcription Factor TonEBP Stimulates Hyperosmolality-Dependent Arginine Vasopressin Gene Expression in the Mouse Hypothalamus.Front Endocrinol (Lausanne). 2021 Mar 16;12:627343. doi: 10.3389/fendo.2021.627343. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33796071 Free PMC article.
References
-
- Ishihara H, Warensjo K, Roberts S, Urban JP. Proteoglycan synthesis in the intervertebral disk nucleus: the role of extracellular osmolality. Am. J. Physiol. 1997;272:C1499–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

