A design-build-test cycle using modeling and experiments reveals interdependencies between upper glycolysis and xylose uptake in recombinant S. cerevisiae and improves predictive capabilities of large-scale kinetic models
- PMID: 28674555
- PMCID: PMC5485749
- DOI: 10.1186/s13068-017-0838-5
A design-build-test cycle using modeling and experiments reveals interdependencies between upper glycolysis and xylose uptake in recombinant S. cerevisiae and improves predictive capabilities of large-scale kinetic models
Abstract
Background: Recent advancements in omics measurement technologies have led to an ever-increasing amount of available experimental data that necessitate systems-oriented methodologies for efficient and systematic integration of data into consistent large-scale kinetic models. These models can help us to uncover new insights into cellular physiology and also to assist in the rational design of bioreactor or fermentation processes. Optimization and Risk Analysis of Complex Living Entities (ORACLE) framework for the construction of large-scale kinetic models can be used as guidance for formulating alternative metabolic engineering strategies.
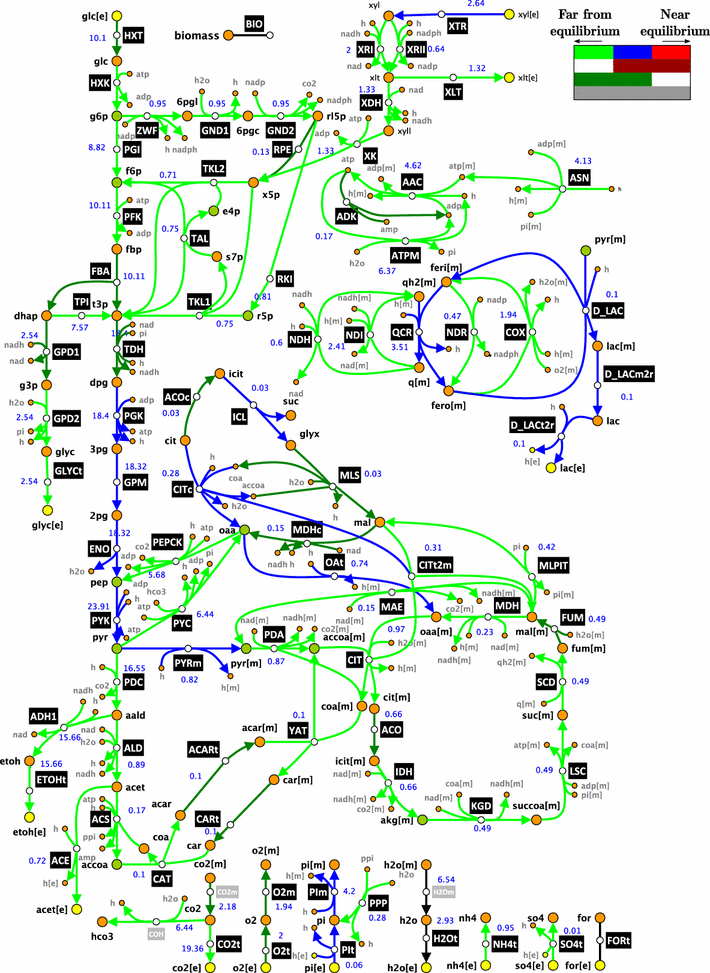
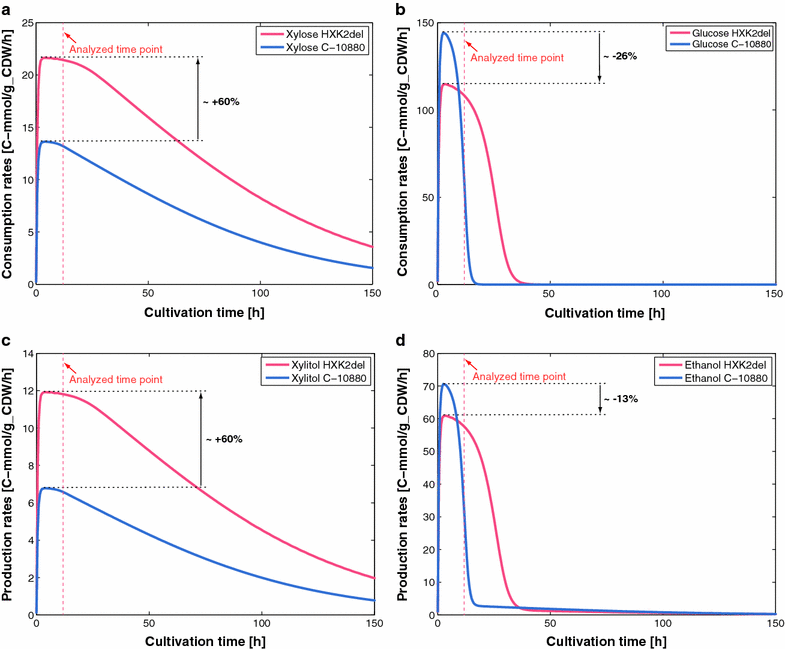
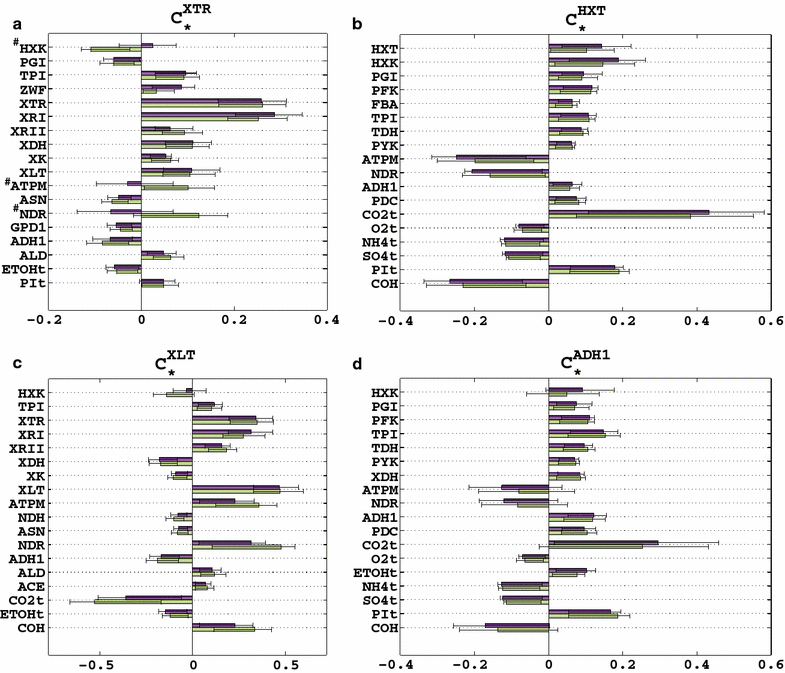
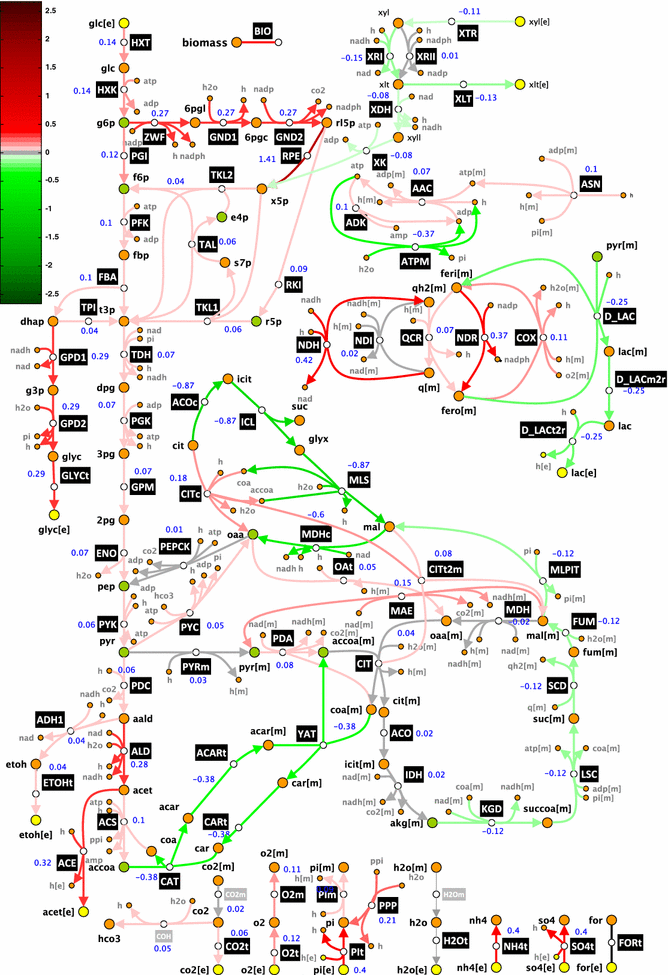
Results: We used ORACLE in a metabolic engineering problem: improvement of the xylose uptake rate during mixed glucose-xylose consumption in a recombinant Saccharomyces cerevisiae strain. Using the data from bioreactor fermentations, we characterized network flux and concentration profiles representing possible physiological states of the analyzed strain. We then identified enzymes that could lead to improved flux through xylose transporters (XTR). For some of the identified enzymes, including hexokinase (HXK), we could not deduce if their control over XTR was positive or negative. We thus performed a follow-up experiment, and we found out that HXK2 deletion improves xylose uptake rate. The data from the performed experiments were then used to prune the kinetic models, and the predictions of the pruned population of kinetic models were in agreement with the experimental data collected on the HXK2-deficient S. cerevisiae strain.
Conclusions: We present a design-build-test cycle composed of modeling efforts and experiments with a glucose-xylose co-utilizing recombinant S. cerevisiae and its HXK2-deficient mutant that allowed us to uncover interdependencies between upper glycolysis and xylose uptake pathway. Through this cycle, we also obtained kinetic models with improved prediction capabilities. The present study demonstrates the potential of integrated "modeling and experiments" systems biology approaches that can be applied for diverse applications ranging from biotechnology to drug discovery.
Keywords: Bioethanol; HXK2 deletion; Hexokinase; Large-scale kinetic models; Metabolic control analysis; S. cerevisiae; Xylose utilization.
Figures




References
-
- Furusawa C, Horinouchi T, Hirasawa T, Shimizu H. Systems metabolic engineering: the creation of microbial cell factories by rational metabolic design and evolution. Adv Biochem Eng Biotechnol. 2013;131:1–23. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

