The influence of storage time and temperature on propofol concentrations in canine blood and plasma
- PMID: 28674652
- PMCID: PMC5494168
- DOI: 10.7717/peerj.3476
The influence of storage time and temperature on propofol concentrations in canine blood and plasma
Abstract
Propofol is an intravenous anesthetic commonly used due to its favorable pharmacokinetic and pharmacodynamic profile. There are discrepancies in the literature about the most appropriate sample for determining propofol concentrations. Although plasma has been used for determining propofol concentrations, whole blood has been the preferred sample. There is also a lack of consistency in the literature on the effect of storage time and temperature on propofol concentrations and this may lead to errors in the design of pharmacokinetic/pharmacodynamics studies. The purpose of this study was to determine the difference in propofol concentrations in whole blood versus plasma and to evaluate the influence of storage time (56 days) and temperature (4 °C, -20 °C, -80 °C) on the stability of propofol concentrations in blood and plasma samples. Results from the study indicate that whole blood and plasma samples containing propofol stored at -80 °C have concentrations as high as or higher than those stored at 4 °C or -20 °C for 56 days; thus, -80 °C is an appropriate temperature for propofol sample storage. Plasma propofol concentrations were consistently higher than whole blood for all three storage temperatures. Consequently, plasma is the most appropriate sample for propofol analysis due to its consistent determinations.
Keywords: Blood; Plasma; Propofol; Stability; Temperature.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
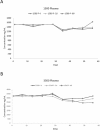
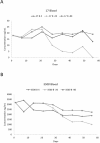
References
-
- Bienert A, Zaba Z, Dyderski S, Ogrodowiz M, Drobnik L. Long-term stability of propofol in human plasma and blood in different storage conditions. Acta Poloniae Pharmaceutica. 2005;62:95–97. - PubMed
-
- Campos S, Monterio J, Balenzuela B, Goncalinho H, Guedes de Pinho P, Fresco P, Felix L, Antunes L. Evidence of different propofol pharmacokinetics under short and prolonged infusion times in rabbits. Basic & Clinical Pharmacology & Toxicology. 2016;118:421–431. doi: 10.1111/bcpt.12521. - DOI - PubMed
-
- Chan K, So APC. The measurement of propofol in human blood samples by Liquid chromatography. Methods and Findings in Experimental and Clinical Pharmacology. 1990;12:135–139. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

