Electrospun poly(N-isopropyl acrylamide)/poly(caprolactone) fibers for the generation of anisotropic cell sheets
- PMID: 28675203
- PMCID: PMC5870125
- DOI: 10.1039/c7bm00324b
Electrospun poly(N-isopropyl acrylamide)/poly(caprolactone) fibers for the generation of anisotropic cell sheets
Abstract
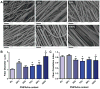
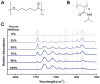
Cell alignment in muscle, nervous tissue, and cartilage is requisite for proper tissue function; however, cell sheeting techniques using the thermosensitive polymer poly(N-isopropyl acrylamide) (PNIPAAm) can only produce anisotropic cell sheets with delicate and resource-intensive modifications. We hypothesized that electrospinning, a relatively simple and inexpensive technique to generate aligned polymer fibers, could be used to fabricate anisotropic PNIPAAm and poly(caprolactone) (PCL) blended surfaces that both support cell viability and permit cell sheet detachment via PNIPAAm dissolution. Aligned electrospun PNIPAAm/PCL fibers (0%, 25%, 50%, 75%, 90%, and 100% PNIPAAm) were electrospun and characterized. Fibers ranged in diameter from 1-3 μm, and all fibers had an orientation index greater than 0.65. Fourier transform infrared spectroscopy was used to confirm the relative content of PNIPAAm and PCL. For advancing water contact angle and mass loss studies, only high PNIPAAm-content fibers (75% and greater) exhibited, temperature-dependent properties like 100% PNIPAAm fibers, whereas 25% and 50% PNIPAAm fibers behaved similarly to PCL-only fibers. 3T3 fibroblasts seeded on all PNIPAAm/PCL fibers had high cell viability and spreading except for the 100% PNIPAAm fibers. Cell sheet detachment by incubation with cold medium was successful only for 90% PNIPAAm fibers, which had a sufficient amount of PCL to allow cell attachment and spreading but not enough to prevent detachment upon PNIPAAm dissolution. This study demonstrates the feasibility of using anisotropic electrospun PNIPAAm/PCL fibers to generate aligned cell sheets that can potentially better recapitulate anisotropic architecture to achieve proper tissue function.
Figures

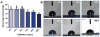


References
-
- Falconnet D, Csucs G, Michelle Grandin H, Textor M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials. 2006;27:3044–3063. - PubMed
-
- Doshi J, Reneker DH. Electrospinning process and applications of electrospun fibers. Conference Record of the 1993 IEEE Industry Applications Society Annual Meeting, 1993. 1993;3:1698–1703. doi:10.1109/ IAS.1993.299067.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

