Intravenous immunoglobulins for epilepsy
- PMID: 28675262
- PMCID: PMC6483482
- DOI: 10.1002/14651858.CD008557.pub3
Intravenous immunoglobulins for epilepsy
Update in
-
Intravenous immunoglobulins for epilepsy.Cochrane Database Syst Rev. 2019 Dec 2;12(12):CD008557. doi: 10.1002/14651858.CD008557.pub4. Cochrane Database Syst Rev. 2019. PMID: 31792946 Free PMC article.
Abstract
Background: Epilepsy is a common neurological condition, with an estimated incidence of 50 per 100,000 persons. People with epilepsy may present with various types of immunological abnormalities, such as low serum immunoglobulin A (IgA) levels, lack of the immunoglobulin G (IgG) subclass and identification of certain types of antibodies. Intravenous immunoglobulin (IVIg) treatment may represent a valuable approach and its efficacy has important implications for epilepsy management. This is an updated version of the original Cochrane review published in Issue 1, 2011.
Objectives: To examine the effects of IVIg on the frequency and duration of seizures, quality of life and adverse effects when used as monotherapy or as add-on treatment for people with epilepsy.
Search methods: For the latest update, we searched the Cochrane Epilepsy Group Specialized Register (2 February 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (2 February 2017), MEDLINE (Ovid, 1946 to 2 February 2017), Web of Science (1898 to 2 February 2017), ISRCTN registry (2 February 2017), WHO International Clinical Trials Registry Platform (ICTRP, 2 February 2017), the US National Institutes of Health ClinicalTrials.gov (2 February 2017), and reference lists of articles.
Selection criteria: Randomized or quasi-randomized controlled trials of IVIg as monotherapy or add-on treatment in people with epilepsy.
Data collection and analysis: Two review authors independently assessed the trials for inclusion and extracted data. We contacted study authors for additional information. Outcomes included percentage of people rendered seizure-free, 50% or greater reduction in seizure frequency, adverse effects, treatment withdrawal and quality of life.
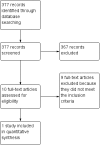
Main results: We included one study (61 participants). The included study was a randomized, double-blind, placebo-controlled, multi-centre trial which compared the treatment efficacy of IVIg as an add-on with a placebo add-on in patients with refractory epilepsy. There was no significant difference between IVIg and placebo in 50% or greater reduction in seizure frequency. The study reported a statistically significant effect for global assessment in favour of IVIg. No adverse effects were demonstrated. We found no randomized controlled trials that investigated the effects of IVIg monotherapy for epilepsy. Overall, the included study was rated as low/unclear risk of bias. Using GRADE methodology, the quality of the evidence was rated as low.
Authors' conclusions: We cannot draw any reliable conclusions regarding the efficacy of IVIg as a treatment for epilepsy. Further randomized controlled trials are needed.
Conflict of interest statement
JG: none known JD: none known YL: none known HN: none known LS: none known GW: none known
Figures









Update of
-
Intravenous immunoglobulins for epilepsy.Cochrane Database Syst Rev. 2011 Jan 19;(1):CD008557. doi: 10.1002/14651858.CD008557.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2017 Jul 04;7:CD008557. doi: 10.1002/14651858.CD008557.pub3. PMID: 21249713 Updated.
References
References to studies included in this review
-
- Rijckevorsel‐Harmant K, Delire M, Schmitz‐Moorman W, Wieser HG. Treatment of refractory epilepsy with intravenous immunoglobulins. Results of the first double‐blind/dose finding clinical study. International Journal of Clinical & Laboratory Research 1994;24(3):162‐6. - PubMed
- Rijckevorsel‐Harmant K, Delire M, Schmitz‐Moormann W, Wieser HG. Refractory epilepsy treated by IVIg in a dose finding, double blind study. Canadian Journal of Neurological Sciences 1993;20(Suppl 4):S181, Abstract no: 6‐11‐15.
References to studies excluded from this review
-
- Arts WF, Aarsen FK, Scheltens‐de Boer M, Catsman‐Berrevoets CE. Landau‐Kleffner syndrome and CSWS syndrome: treatment with intravenous immunoglobulins. Epilepsia 2009;50 Suppl 7:55‐8. - PubMed
-
- Bien CG, Tiemeier H, Sassen R, Kuczaty S, Urbach H, Lehe M, et al. Rasmussen encephalitis: incidence and course under randomized therapy with tacrolimus or intravenous immunoglobulins. Epilepsia 2013;54(3):543‐50. - PubMed
-
- Billiau AD, Witters P, Ceulemans B, Kasran A, Wouters C, Lagae L. Intravenous immunoglobulins in refractory childhood‐onset epilepsy: effects on seizure frequency, EEG activity, and cerebrospinal fluid cytokine profile. Epilepsia 2007;48(9):1739‐49. - PubMed
-
- Geva‐Dayan K, Shorer Z, Menascu S, Linder I, Goldberg‐Stern H, Heyman E, et al. Immunoglobulin treatment for severe childhood epilepsy. Pediatric Neurology 2012;46(6):375‐81. - PubMed
-
- Gross‐Tsur V, Shalev RS, Kazir E, Engelhard D, Amir N. Intravenous high‐dose gammaglobulins for intractable childhood epilepsy. Acta Neurologica Scandinavica 1993;88(3):204‐9. - PubMed
References to ongoing studies
-
- NCT02697292. IVIG in patients with VGKC Ab associated autoimmune epilepsy [A randomized double blind placebo controlled study of intravenous immunoglobulin (IVIG) patients with voltage gated potassium channel complex (VGKC) antibody associated autoimmune epilepsy]. https://clinicaltrials.gov/ct2/show/record/NCT02697292 (first received 19 February 2016).
Additional references
-
- Ariizumi M, Baba K, Hibio S, Shiihara H, Michihiro N, Ogawa K, et al. Immunoglobulin therapy in the West syndrome. Brain and Development 1987;9(4):422‐5. - PubMed
-
- Bedini R, Feo MR, Orano A, Rocchi L. Effects of gamma‐globulin therapy in severely epileptic children. Epilepsia 1985;26(1):98‐102. - PubMed
-
- Billiau AD, Wouters CH, Lagae LG. Epilepsy and the immune system: is there a link?. European Journal of Paediatric Neurology 2005;9(1):29‐42. - PubMed
-
- Bingel U, Pinter JD, Sotero de Menezes M, Rho JM. Intravenous immunoglobulin as adjunctive therapy for juvenile spasms. Journal of Child Neurology 2003;18(6):379‐82. - PubMed
-
- Bostantjopoulou S, Hatzizisi O, Argyropoulou O, Andreadis S, Deligiannis K, Kantaropoulou M, et al. Immunological parameters in patients with epilepsy. Functional Neurology 1994;9(1):11‐15. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

