Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy
- PMID: 28676095
- PMCID: PMC5496237
- DOI: 10.1186/s13024-017-0195-7
Toll-like receptor 4 stimulation with monophosphoryl lipid A ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal α-synucleinopathy
Abstract
Background: Alpha-synuclein (α-syn) aggregation represents the pathological hallmark of α-synucleinopathies like Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). Toll-like receptors (TLRs) are a family of highly conserved molecules that recognize pathogen-associated molecular patterns and define the innate immunity response. It was previously shown that TLR4 plays a role in the clearance of α-syn, suggesting that TLR4 up-regulation in microglia may be a natural mechanism to improve the clearance of α-syn. However, administration of TLR4 ligands could also lead to dangerous adverse effects associated with the induction of toxic inflammatory responses. Monophosphoryl lipid A (MPLA) is a TLR4 selective agonist and a potent inducer of phagocytosis which does not trigger strong toxic inflammatory responses as compared to lipopolysaccharide (LPS). We hypothesize that MPLA treatment will lead to increased clearance of α-syn inclusions in the brain of transgenic mice overexpressing α-syn in oligodendrocytes under the proteolipid protein promoter (PLP-α-syn mouse model of MSA), without triggering toxic cytokine release, thus leading to a general amelioration of the pathology.
Methods: Six month old PLP-α-syn mice were randomly allocated to four groups and received weekly intraperitoneal injections of MPLA (50 or 100 μg), LPS or vehicle. After a 12-week treatment period, motor behavior was assessed with the pole test. Brains and plasma samples were collected for neuropathological and immunological analysis.
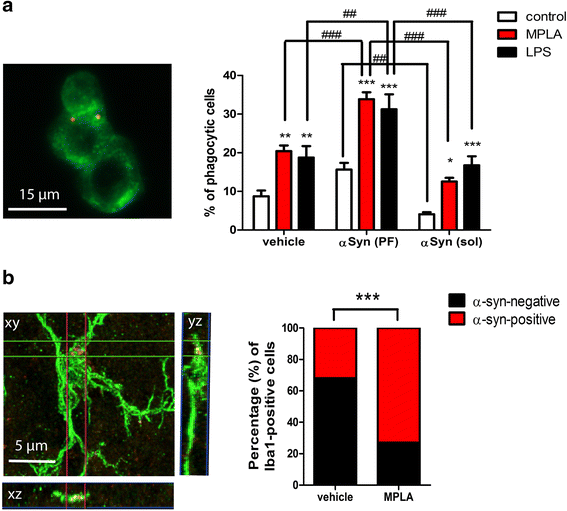
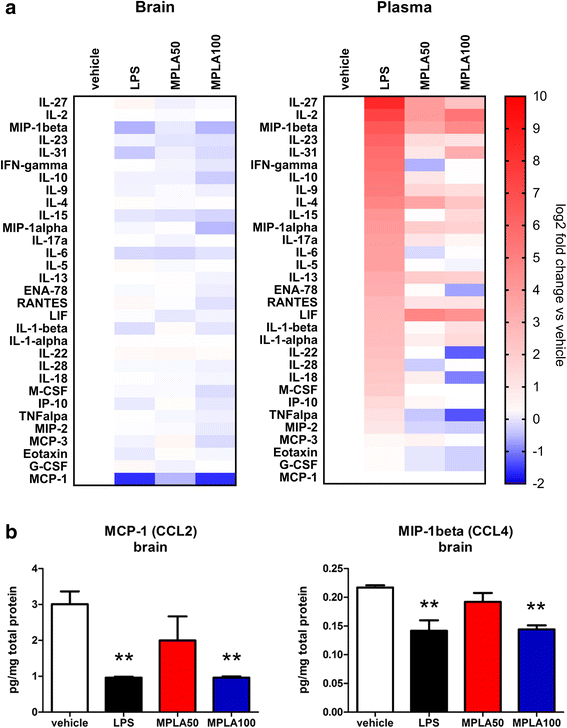
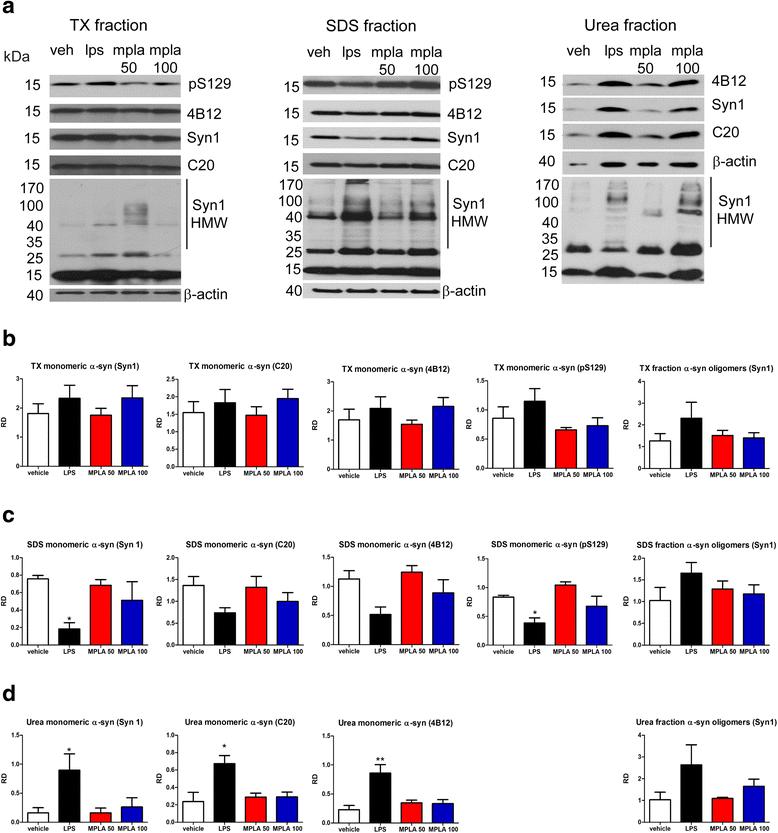
Results: Chronic systemic MPLA treatment of PLP-α-syn mice led to increased uptake of α-syn by microglial cells, a significant motor improvement, rescue of nigral dopaminergic and striatal neurons and region-specific reduction of the density of oligodendroglial α-syn cytoplasmic inclusions in the absence of a marked systemic inflammatory response.
Conclusion: Our findings demonstrate beneficial effects of chronic MPLA treatment in transgenic PLP-α-syn mice. MPLA appears to be an attractive therapeutic candidate for disease modification trials in MSA and related α-synucleinopathies.
Keywords: Inclusion pathology; Monophosphoryl lipid A; Neuroinflammation; Toll-like receptor; α-synuclein.
Conflict of interest statement
Authors’ information
SV, VR, GKW and NS are from the Medical University of Innsbruck, Department of Neurology, 6020 Innsbruck, Austria. Ap and LS are from the Laboratory of Neurodegenerative Diseases, Division of Basic Neurosciences, Biomedical Research Foundation of the Academy of Athens, Greece.
Ethics approval
All research involving animals has been approved by the Ethics Board at the Federal Ministry of Science and Research, Austria (permission BMWFW-66.011/0122-WF/V/3b/2014). Human subjects’ consent to participate is not applicable.
Consent for publication
All contributing authors have given their consent for the publication of this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies.Acta Neuropathol Commun. 2018 Jan 3;6(1):2. doi: 10.1186/s40478-017-0504-y. Acta Neuropathol Commun. 2018. PMID: 29298733 Free PMC article.
-
ATH434 Reduces α-Synuclein-Related Neurodegeneration in a Murine Model of Multiple System Atrophy.Mov Disord. 2021 Nov;36(11):2605-2614. doi: 10.1002/mds.28714. Epub 2021 Jul 8. Mov Disord. 2021. PMID: 34236731
-
Reducing C-terminal truncation mitigates synucleinopathy and neurodegeneration in a transgenic model of multiple system atrophy.Proc Natl Acad Sci U S A. 2016 Aug 23;113(34):9593-8. doi: 10.1073/pnas.1609291113. Epub 2016 Aug 1. Proc Natl Acad Sci U S A. 2016. PMID: 27482103 Free PMC article.
-
Review: Novel treatment strategies targeting alpha-synuclein in multiple system atrophy as a model of synucleinopathy.Neuropathol Appl Neurobiol. 2016 Feb;42(1):95-106. doi: 10.1111/nan.12312. Neuropathol Appl Neurobiol. 2016. PMID: 26924723 Free PMC article. Review.
-
Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
Cited by
-
Lysosomal storage, impaired autophagy and innate immunity in Gaucher and Parkinson's diseases: insights for drug discovery.Philos Trans R Soc Lond B Biol Sci. 2024 Apr 8;379(1899):20220381. doi: 10.1098/rstb.2022.0381. Epub 2024 Feb 19. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 38368939 Free PMC article. Review.
-
The immune system in Parkinson's disease: what we know so far.Brain. 2024 Oct 3;147(10):3306-3324. doi: 10.1093/brain/awae177. Brain. 2024. PMID: 38833182 Free PMC article. Review.
-
Recent Advances in Clinical Trials in Multiple System Atrophy.Curr Neurol Neurosci Rep. 2024 Apr;24(4):95-112. doi: 10.1007/s11910-024-01335-0. Epub 2024 Feb 28. Curr Neurol Neurosci Rep. 2024. PMID: 38416311 Review.
-
Microglia: Key Players in Retinal Ageing and Neurodegeneration.Front Cell Neurosci. 2022 Mar 17;16:804782. doi: 10.3389/fncel.2022.804782. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35370560 Free PMC article. Review.
-
Intranasal Monophosphoryl Lipid a Administration Ameliorates depression-like Behavior in Chronically Stressed Mice Through Stimulation of Microglia.Neurochem Res. 2023 Oct;48(10):3160-3176. doi: 10.1007/s11064-023-03974-0. Epub 2023 Jun 26. Neurochem Res. 2023. PMID: 37358676
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

