How cells channel their stress: Interplay between Piezo1 and the cytoskeleton
- PMID: 28676421
- PMCID: PMC6070642
- DOI: 10.1016/j.semcdb.2017.06.018
How cells channel their stress: Interplay between Piezo1 and the cytoskeleton
Abstract
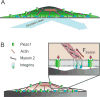
Cells constantly encounter mechanical stimuli in their environment, such as dynamic forces and mechanical features of the extracellular matrix. These mechanical cues are transduced into biochemical signals, and integrated with genetic and chemical signals to modulate diverse physiological processes. Cells also actively generate forces to internally transport cargo, to explore the physical properties of their environment and to spatially position themselves and other cells during development. Mechanical forces are therefore central to development, homeostasis, and repair. Several molecular and biophysical strategies are utilized by cells for detecting and generating mechanical forces. Here we discuss an important class of molecules involved in sensing and transducing mechanical forces - mechanically-activated ion channels. We focus primarily on the Piezo1 ion channel, and examine its relationship with the cellular cytoskeleton.
Keywords: Calcium signaling; Cytoskeleton; Mechanically-activated ion channels; Mechanotransduction; Piezo1; Traction forces.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


References
-
- Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 2010;64:313–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

